Identification of blood vascular endothelial stem cells by the expression of protein C receptor
- PMID: 27364685
- PMCID: PMC5113308
- DOI: 10.1038/cr.2016.85
Identification of blood vascular endothelial stem cells by the expression of protein C receptor
Abstract
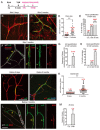
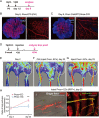
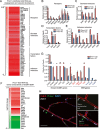
Vascular growth and remodeling are dependent on the generation of new endothelial cells from stem cells and the involvement of perivascular cells to maintain vessel integrity and function. The existence and cellular identity of vascular endothelial stem cells (VESCs) remain unclear. The perivascular pericytes in adult tissues are thought to arise from the recruitment and differentiation of mesenchymal progenitors during early development. In this study, we identified Protein C receptor-expressing (Procr+) endothelial cells as VESCs in multiple tissues. Procr+ VESCs exhibit robust clonogenicity in culture, high vessel reconstitution efficiency in transplantation, long-term clonal expansion in lineage tracing, and EndMT characteristics. Moreover, Procr+ VESCs are bipotent, giving rise to de novo formation of endothelial cells and pericytes. This represents a novel origin of pericytes in adult angiogenesis, reshaping our understanding of blood vessel development and homeostatic process. Our study may also provide a more precise therapeutic target to inhibit pathological angiogenesis and tumor growth.
Figures
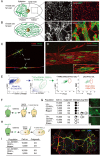
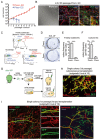
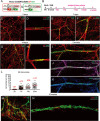
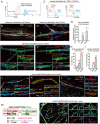
Comment in
-
Vascular Procr+ stem cells: Finding new branches while looking for the roots.Cell Res. 2016 Oct;26(10):1071-1072. doi: 10.1038/cr.2016.91. Epub 2016 Aug 2. Cell Res. 2016. PMID: 27481542 Free PMC article.
References
-
- Diaz-Flores L, Gutierrez R, Varela H, Rancel N, Valladares F. Microvascular pericytes: a review of their morphological and functional characteristics. Histol Histopathol 1991; 6:269–286. - PubMed
-
- Pallone TL, Silldorff EP. Pericyte regulation of renal medullary blood flow. Exp Nephrol 2001; 9:165–170. - PubMed
-
- Lindahl P, Johansson BR, Leveen P, Betsholtz C. Pericyte loss and microaneurysm formation in PDGF-B-deficient mice. Science 1997; 277:242–245. - PubMed
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Molecular Biology Databases
Miscellaneous

