Infiltrating T lymphocytes reduce myeloid phagocytosis activity in synucleinopathy model
- PMID: 27364890
- PMCID: PMC4929755
- DOI: 10.1186/s12974-016-0632-5
Infiltrating T lymphocytes reduce myeloid phagocytosis activity in synucleinopathy model
Abstract
Background: Synucleinopathies comprise a group of neurodegenerative diseases associated with abnormal accumulation of α-synuclein. One of the key factors that contribute to the progression of synucleinopathies is neuroinflammation. However, the role of lymphocytes in synucleinopathies like Parkinson's disease (PD) remains largely unclear.
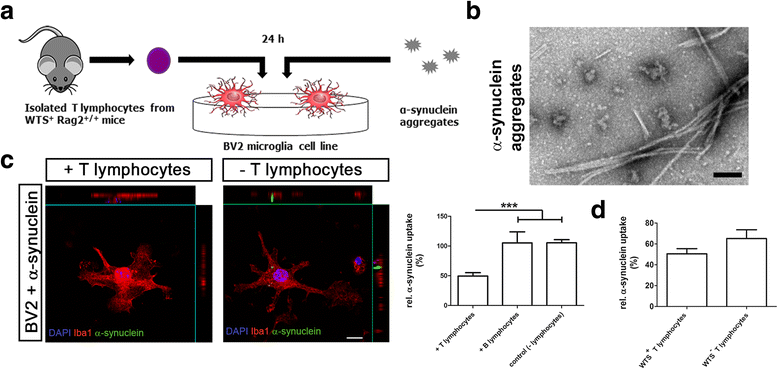
Methods: To investigate how lymphocytes impact synucleinopathies, human wild-type α-synuclein (WTS) transgenic mice were crossed with mice lacking mature lymphocytes (Rag2(-/-)). In this in vivo model, we quantified α-synuclein aggregation in the substantia nigra (SN) and striatum and determined the numbers of innate and adaptive immune cells in the central nervous system (CNS). The activation state of resident and infiltrated CNS myeloid cells (M1 vs. M2) was further classified by gene and protein expression analyses. The impact of T and B lymphocytes on the phagocytic activity of microglia in the presence of α-synuclein aggregates was addressed in BV2 microglia in vitro.
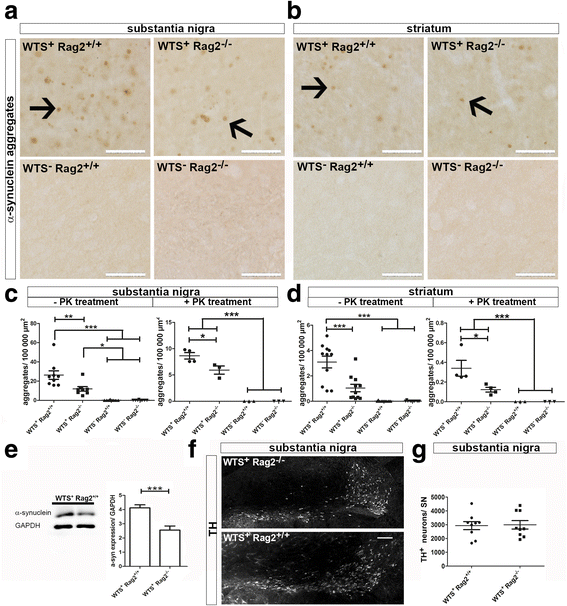
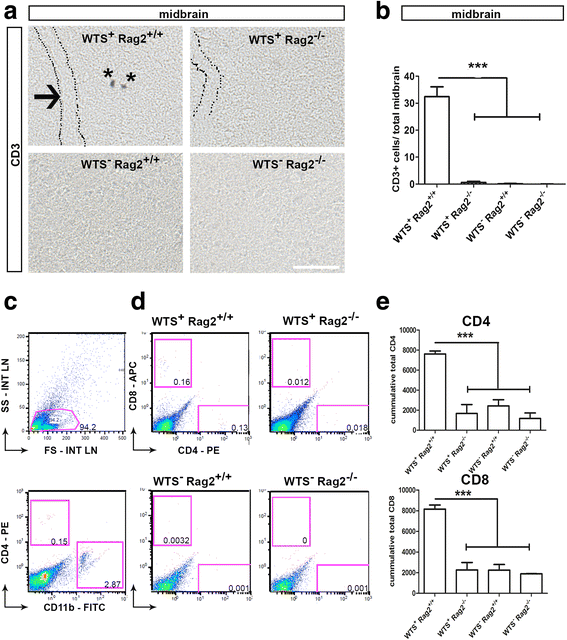
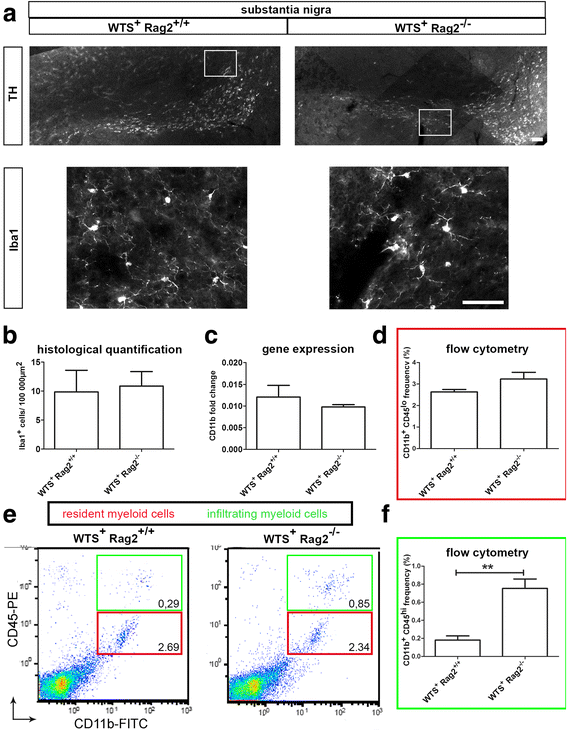
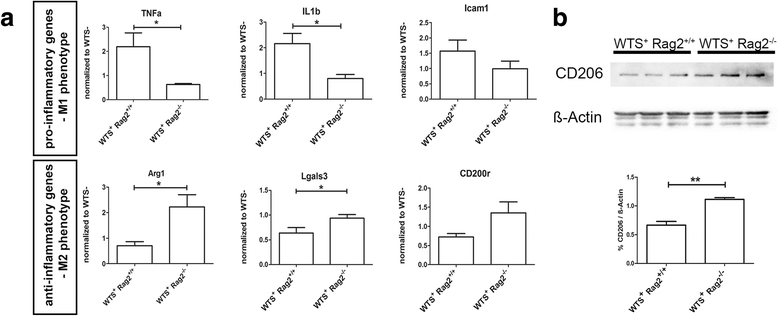
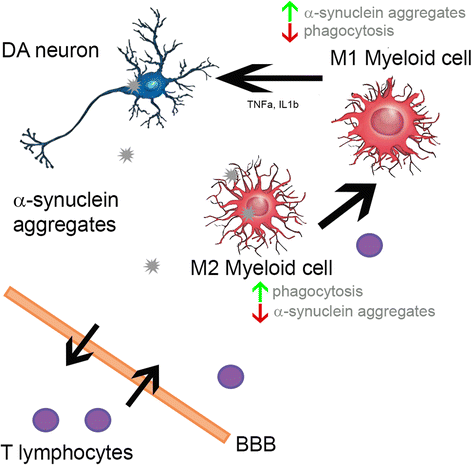
Results: Compared to WTS(+) Rag2(+/+) mice, where T but not B lymphocytes infiltrated the CNS, decreased amounts of α-synuclein aggregates were found in WTS(+) Rag2(-/-) mice devoid of mature lymphocytes. The presence of T lymphocytes did not alter the number of Iba1(+) microglia but increased the frequency of the CD11b(+) CD45(hi) population in the CNS, indicative of an increased number of infiltrated macrophages. Moreover, the M1 phenotype was more prominent in WTS(+) Rag2(+/+) mice, whereas the M2 activation state was dominating in the absence of lymphocytes in WTS(+) Rag2(-/-) mice. In vitro, in the presence of T but not B lymphocytes, significantly less α-synuclein was phagocytosed by BV2 microglia, further supporting the prevalence of the M1 phenotype in the presence of T lymphocytes.
Conclusions: Peripheral T lymphocytes strongly contribute to increased α-synuclein pathology via modulation of CNS myeloid cell function. In the presence of T lymphocytes, microglia phagocytosis of aggregated α-synuclein is reduced, which increases the severity of synucleinopathy.
Keywords: Adaptive immune system; Parkinson’s disease; Rag2; mThy1 WTS model.
Figures
References
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Research Materials
Miscellaneous

