Haptoglobin increases the vulnerability of CD163-expressing neurons to hemoglobin
- PMID: 27364920
- PMCID: PMC5304903
- DOI: 10.1111/jnc.13720
Haptoglobin increases the vulnerability of CD163-expressing neurons to hemoglobin
Abstract
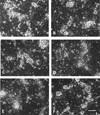
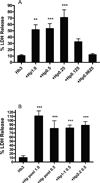
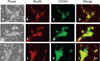
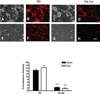
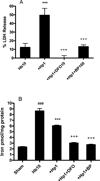
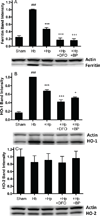
Haptoglobin (Hp) binds hemoglobin (Hb) with high affinity and provides the primary defense against its toxicity after intravascular hemolysis. Neurons are exposed to extracellular Hb after CNS hemorrhage, and a therapeutic effect of Hp via Hb sequestration has been hypothesized. In this study, we tested the hypothesis that Hp protects neurons from Hb in primary mixed cortical cell cultures. Treatment with low micromolar concentrations of human Hb for 24 h resulted in loss of 10-20% of neurons without injuring glia. Concomitant treatment with Hp surprisingly increased neuronal loss five-sevenfold, with similar results produced by Hp 1-1 and 2-2 phenotypes. Consistent with a recent in vivo observation, neurons expressed the CD163 receptor for Hb and the Hb-Hp complex in these cultures. Hp reduced overall Hb uptake, directed it away from the astrocyte-rich CD163-negative glial monolayer, and decreased induction of the iron-binding protein ferritin. Hb-Hp complex neuronal toxicity, like that of Hb per se, was iron-dependent and reduced by deferoxamine and 2,2' bipyridyl. These results suggest that Hp increases the vulnerability of CD163+ neurons to Hb by permitting Hb uptake while attenuating the protective response of ferritin induction by glial cells. Cover Image for this issue: doi: 10.1111/jnc.13342.
Keywords: intracerebral hemorrhage; iron; neurotoxicity; stroke; subarachnoid hemorrhage.
© 2016 International Society for Neurochemistry.
Conflict of interest statement
The authors report no conflicts of interest.
Figures


References
-
- Arosio P, Levi S. Ferritin, iron homeostasis, and oxidative damage. Free Radic Biol Med. 2002;33:457–463. - PubMed
-
- ARUP. Haptoglobin: ARUP Lab Tests. 2016
-
- Borsody M, Burke A, Coplin W, Miller-Lotan R, Levy A. Haptoglobin and the development of cerebral artery vasospasm after subarachnoid hemorrhage. Neurology. 2006;66:634–640. - PubMed
-
- Brewer GJ. Serum-free B27/neurobasal medium supports differentiated growth of neurons from the striatum, substantia nigra, septum, cerebral cortex, cerebellum, and dentate gyrus. J Neurosci Res. 1995;42:674–683. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Research Materials
Miscellaneous

