Perturbed proteostasis in autism spectrum disorders
- PMID: 27365114
- PMCID: PMC5215415
- DOI: 10.1111/jnc.13723
Perturbed proteostasis in autism spectrum disorders
Abstract
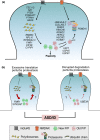
Dynamic changes in synaptic strength rely on de novo protein synthesis and protein degradation by the ubiquitin proteasome system (UPS). Disruption of either of these cellular processes will result in significant impairments in synaptic plasticity and memory formation. Mutations in several genes encoding regulators of mRNA translation and members of the UPS have been associated with an increased risk for the development of autism spectrum disorders. It is possible that these mutations result in a similar imbalance in protein homeostasis (proteostasis) at the synapse. This review will summarize recent work investigating the role of the UPS in synaptic plasticity at glutamatergic synapses, and propose that dysfunctional proteostasis is a common consequence of several genetic mutations linked to autism spectrum disorders. Dynamic changes in synaptic strength rely on de novo protein synthesis and protein degradation by the ubiquitin proteasome system (UPS). Disruption of either of these cellular processes will result in significant impairments in synaptic plasticity and memory formation. Mutations in several genes encoding regulators of mRNA translation (i.e. FMR1) and protein degradation (i.e. UBE3A) have been associated with an increased risk for autism spectrum disorders and intellectual disability (ASD/ID). These mutations similarly disrupt protein homeostasis (proteostasis). Compensatory changes that reset the rate of proteostasis may contribute to the neurological symptoms of ASD/ID. This review summarizes recent work investigating the role of the UPS in synaptic plasticity at glutamatergic synapses, and proposes that dysfunctional proteostasis is a common consequence of several genetic mutations linked to ASD. This article is part of a mini review series: "Synaptic Function and Dysfunction in Brain Diseases".
Keywords: ASD/ID; autism; proteasome; synaptic plasticity; translation; ubiquitin.
© 2016 The Authors. Journal of Neurochemistry published by John Wiley & Sons Ltd on behalf of International Society for Neurochemistry.
Figures
References
-
- Alfieri P., Piccini G., Caciolo C. et al (2014) Behavioral profile in RASopathies. Am. J. Med. Genet. A 164A, 934–942. - PubMed
-
- Alfieri P., Caciolo C., Piccini G. et al (2015) Behavioral phenotype in Costello syndrome with atypical mutation: a case report. Am. J. Med. Genet. B Neuropsychiatr. Genet. 168B, 66–71. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical

