Detectable Risks in Studies of the Fetal Benefits of Maternal Influenza Vaccination
- PMID: 27365363
- PMCID: PMC4967598
- DOI: 10.1093/aje/kww048
Detectable Risks in Studies of the Fetal Benefits of Maternal Influenza Vaccination
Abstract
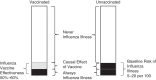
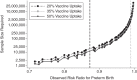
Maternal influenza vaccination prevents influenza illness in both mothers and newborns. Results from some recent studies have suggested that influenza vaccination might also prevent adverse pregnancy outcomes, such as preterm birth. However, it is challenging to conduct epidemiologic studies to evaluate the benefits to the fetus of maternal influenza vaccination because the causal benefit of vaccination is likely only experienced by the small fraction of the cohort in whom influenza illness is prevented by vaccination. The plausibility of detecting true differences in risks between groups under such conditions is rarely discussed. We aimed to inform the interpretation of studies in which the fetal benefits of maternal influenza vaccination are evaluated by estimating detectable risk ratios and necessary sample sizes for different study scenarios. Estimates of rates of influenza illness, vaccine effectiveness, vaccine uptake, and preterm birth and of the association of influenza illness with preterm birth were identified from the published literature. We calculated detectable risk ratios for preterm birth in vaccinated versus unvaccinated women and the associated sample size requirements. Our results demonstrated that under most scenarios, plausible differences between groups will be extremely challenging to detect (risk ratios for preterm birth of 0.9 to 1.0) and will require sample sizes infeasible for prospective epidemiologic research. This suggests that the large fetal benefits from influenza vaccination observed in epidemiologic studies are unlikely to be causal.
Keywords: immunization; influenza illness; influenza vaccine; pregnancy; pregnancy complication; preterm birth; sample size; statistical power.
© The Author 2016. Published by Oxford University Press on behalf of the Johns Hopkins Bloomberg School of Public Health.
Figures
Comment in
-
Hutcheon et al. Respond to "Maternal Influenza Immunization and Birth Outcomes".Am J Epidemiol. 2016 Dec 1;184(11):793-795. doi: 10.1093/aje/kww111. Epub 2016 Oct 26. Am J Epidemiol. 2016. PMID: 27784656 Free PMC article. No abstract available.
-
Maternal Influenza Immunization and Adverse Birth Outcomes: Using Data and Practice to Inform Theory and Research Design.Am J Epidemiol. 2016 Dec 1;184(11):789-792. doi: 10.1093/aje/kww110. Epub 2016 Oct 26. Am J Epidemiol. 2016. PMID: 27784657 Free PMC article.
-
THE AUTHORS REPLY.Am J Epidemiol. 2017 May 1;185(9):861-862. doi: 10.1093/aje/kww203. Am J Epidemiol. 2017. PMID: 28369158 Free PMC article. No abstract available.
-
RE: "DETECTABLE RISKS IN STUDIES OF THE FETAL BENEFITS OF MATERNAL INFLUENZA VACCINATION".Am J Epidemiol. 2017 May 1;185(9):860-861. doi: 10.1093/aje/kww202. Am J Epidemiol. 2017. PMID: 28369245 No abstract available.
References
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical

