The Molecular Chaperone Hsc70 Interacts with Tyrosine Hydroxylase to Regulate Enzyme Activity and Synaptic Vesicle Localization
- PMID: 27365397
- PMCID: PMC5016148
- DOI: 10.1074/jbc.M116.728782
The Molecular Chaperone Hsc70 Interacts with Tyrosine Hydroxylase to Regulate Enzyme Activity and Synaptic Vesicle Localization
Abstract
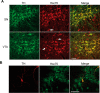
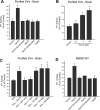
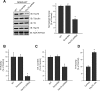
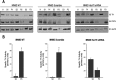
We previously reported that the vesicular monoamine transporter 2 (VMAT2) is physically and functionally coupled with Hsc70 as well as with the dopamine synthesis enzymes tyrosine hydroxylase (TH) and aromatic amino acid decarboxylase, providing a novel mechanism for dopamine homeostasis regulation. Here we expand those findings to demonstrate that Hsc70 physically and functionally interacts with TH to regulate the enzyme activity and synaptic vesicle targeting. Co-immunoprecipitation assays performed in brain tissue and heterologous cells demonstrated that Hsc70 interacts with TH and aromatic amino acid decarboxylase. Furthermore, in vitro binding assays showed that TH directly binds the substrate binding and carboxyl-terminal domains of Hsc70. Immunocytochemical studies indicated that Hsc70 and TH co-localize in midbrain dopaminergic neurons. The functional significance of the Hsc70-TH interaction was then investigated using TH activity assays. In both dopaminergic MN9D cells and mouse brain synaptic vesicles, purified Hsc70 facilitated an increase in TH activity. Neither the closely related protein Hsp70 nor the unrelated Hsp60 altered TH activity, confirming the specificity of the Hsc70 effect. Overexpression of Hsc70 in dopaminergic MN9D cells consistently resulted in increased TH activity whereas knockdown of Hsc70 by short hairpin RNA resulted in decreased TH activity and dopamine levels. Finally, in cells with reduced levels of Hsc70, the amount of TH associated with synaptic vesicles was decreased. This effect was rescued by addition of purified Hsc70. Together, these data demonstrate a novel interaction between Hsc70 and TH that regulates the activity and localization of the enzyme to synaptic vesicles, suggesting an important role for Hsc70 in dopamine homeostasis.
Keywords: Hsc70; complex; dopamine; enzyme; protein targeting; synapse.
© 2016 by The American Society for Biochemistry and Molecular Biology, Inc.
Figures




References
-
- Skagerberg G., Lindvall O., and Björklund A. (1984) Origin, course and termination of the mesohabenular dopamine pathway in the rat. Brain Res. 307, 99–108 - PubMed
-
- Björklund A., and Dunnett S. B. (2007) Dopamine neuron systems in the brain: an update. Trends Neurosci. 30, 194–202 - PubMed
-
- Arias-Carrion O., and Poppel E. (2007) Dopamine, learning, and reward-seeking behavior. Acta Neurobiol. Exp. 67, 481–488 - PubMed
-
- Greengard P. (2001) The neurobiology of dopamine signaling. Biosci. Rep. 21, 247–269 - PubMed
-
- Carlsson A. (1987) Development of new pharmacological approaches in Parkinson's disease. Adv. Neurol. 45, 513–518 - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Research Materials
Miscellaneous

