Immune modulation by MANF promotes tissue repair and regenerative success in the retina
- PMID: 27365452
- PMCID: PMC5270511
- DOI: 10.1126/science.aaf3646
Immune modulation by MANF promotes tissue repair and regenerative success in the retina
Abstract
Regenerative therapies are limited by unfavorable environments in aging and diseased tissues. A promising strategy to improve success is to balance inflammatory and anti-inflammatory signals and enhance endogenous tissue repair mechanisms. Here, we identified a conserved immune modulatory mechanism that governs the interaction between damaged retinal cells and immune cells to promote tissue repair. In damaged retina of flies and mice, platelet-derived growth factor (PDGF)-like signaling induced mesencephalic astrocyte-derived neurotrophic factor (MANF) in innate immune cells. MANF promoted alternative activation of innate immune cells, enhanced neuroprotection and tissue repair, and improved the success of photoreceptor replacement therapies. Thus, immune modulation is required during tissue repair and regeneration. This approach may improve the efficacy of stem-cell-based regenerative therapies.
Copyright © 2016, American Association for the Advancement of Science.
Figures
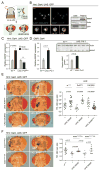

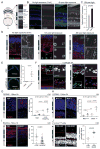
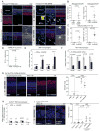


Comment in
-
NEUROREGENERATION. Promoting CNS repair.Science. 2016 Jul 1;353(6294):30-1. doi: 10.1126/science.aag3327. Science. 2016. PMID: 27365439 No abstract available.
References
-
- Trounson A, McDonald C. Stem Cell Therapies in Clinical Trials: Progress and Challenges. Cell stem cell. 2015 Jul 2;17:11. - PubMed
-
- Forbes SJ, Rosenthal N. Preparing the ground for tissue regeneration: from mechanism to therapy. Nature medicine. 2014 Aug;20:857. - PubMed
-
- Reynolds J, Lamba DA. Human embryonic stem cell applications for retinal degenerations. Experimental eye research. 2014 Jun;123:151. - PubMed
-
- Gupta N, Brown KE, Milam AH. Activated microglia in human retinitis pigmentosa, late-onset retinal degeneration, and age-related macular degeneration. Experimental eye research. 2003 Apr;76:463. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases
Miscellaneous

