Preischemic Administration of Nonexpanded Adipose Stromal Vascular Fraction Attenuates Acute Renal Ischemia/Reperfusion Injury and Fibrosis
- PMID: 27365485
- PMCID: PMC4996434
- DOI: 10.5966/sctm.2015-0223
Preischemic Administration of Nonexpanded Adipose Stromal Vascular Fraction Attenuates Acute Renal Ischemia/Reperfusion Injury and Fibrosis
Abstract
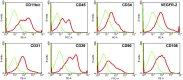
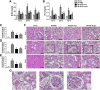
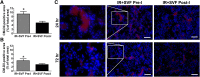
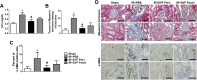
: Ischemia/reperfusion (IR)-induced acute kidney injury (AKI) is a common clinical syndrome. Stem/progenitor cell therapy is a promising option to foster the intrinsic capacity for kidney regeneration. However, there are still several challenges to be resolved, including the potential risks during cell culture, low retention rate after transplantation, and unclear effect on the progression of chronic kidney disease (CKD). Recently, nonexpanded adipose stromal vascular fraction (SVF) has been regarded as an attractive cell source for cell-based therapy. Preconditioning with ischemia has been suggested as a useful method to promote the retention and survival of transplanted cells in vivo. In this study, freshly isolated autologous SVF was transplanted to the kidney of rats before ischemia, and then an IR-induced AKI model was established. Postischemic administration of SVF to the kidney was performed after renal IR injury was induced. A higher cell retention rate was detected in the preischemic group. Preischemic administration of SVF showed stronger functional and morphologic protection from renal IR injury than postischemic administration, through enhancing tubular cell proliferation and reducing apoptosis. Progression of kidney fibrosis was also significantly delayed by preischemic administration of SVF, which exhibited stronger inhibition of transforming growth factor-β1-induced epithelia-mesenchymal transition and microvascular rarefaction. In addition, in vitro study showed that prehypoxic administration of SVF could significantly promote the proliferation, migration, and survival of hypoxic renal tubular epithelial cells. In conclusion, our study demonstrated that preischemic administration of nonexpanded adipose SVF protected the kidney from both acute IR injury and long-term risk of developing CKD.
Significance: Renal ischemia/reperfusion (IR) injury is a common clinical syndrome. Cell-based therapy provides a promising option to promote renal repair after IR injury. However, several challenges still remain because of the potential risks during cell culture, low retention rate after transplantation, and unclear effect on the progression of chronic kidney disease. Stromal vascular fraction (SVF) is considered as an attractive cell source. This study demonstrated that preischemic administration of uncultured SVF could increase cell retention and then improve renal function and structure at both early and long-term stage after IR, which may provide a novel therapeutic approach for IR injury.
Keywords: Adipose stem cells; Autologous stem cell transplantation; Cellular therapy; Ischemia/reperfusion; Renal; Stromal vascular fraction.
©AlphaMed Press.
Figures



References
-
- Liaño F, Pascual J, Madrid Acute Renal Failure Study Group Epidemiology of acute renal failure: A prospective, multicenter, community-based study. Kidney Int. 1996;50:811–818. - PubMed
-
- Sharfuddin AA, Molitoris BA. Pathophysiology of ischemic acute kidney injury. Nat Rev Nephrol. 2011;7:189–200. - PubMed
-
- Leung KC, Tonelli M, James MT. Chronic kidney disease following acute kidney injury-risk and outcomes. Nat Rev Nephrol. 2013;9:77–85. - PubMed
MeSH terms
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical

