Effect of Hydroxychloroquine Treatment on Dry Eyes in Subjects with Primary Sjögren's Syndrome: a Double-Blind Randomized Control Study
- PMID: 27366013
- PMCID: PMC4901007
- DOI: 10.3346/jkms.2016.31.7.1127
Effect of Hydroxychloroquine Treatment on Dry Eyes in Subjects with Primary Sjögren's Syndrome: a Double-Blind Randomized Control Study
Abstract
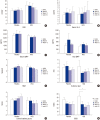
The effect of hydroxychloroquine (HCQ) on dry eye has not been fully determined. This study aimed to compare the 12-week efficacy of HCQ medication with that of a placebo in the management of dry eye in primary Sjögren's syndrome (pSS). A double-blind, randomized control study was conducted in 39 pSS subjects from May 2011 through August 2013. pSS was diagnosed based on the classification criteria of the American-European Consensus Group. Subjects received 300 mg of HCQ or placebo once daily for 12 weeks and were evaluated at baseline, 6, and 12 weeks, with a re-visit at 16 weeks after drug discontinuance. The fluorescein staining score, Schirmer test score, tear film break-up time (TBUT), and ocular surface disease index (OSDI) were measured, and tears and blood were collected for ESR, IL-6, IL-17, B-cell activating factor (BAFF), and Th17 cell analysis. Color testing was performed and the fundus was examined to monitor HCQ complications. Twenty-six subjects completed the follow-up. The fluorescein staining score and Schirmer test score did not differ significantly. The OSDI improved with medication in the HCQ group but was not significantly different between the groups. TBUT, serum IL-6, ESR, serum and tear BAFF, and the proportion of Th17 cells did not change in either group. HCQ at 300 mg daily for 12 weeks has no apparent clinical benefit for dry eye and systemic inflammation in pSS (ClinicalTrials.gov. NCT01601028).
Keywords: Double-Blind Method; Dry Eye Syndromes; Hydroxychloroquine; Prospective Studies; Sjögren's Syndrome.
Conflict of interest statement
Figures

References
-
- Ramos-Casals M, Tzioufas AG, Stone JH, Sisó A, Bosch X. Treatment of primary Sjögren syndrome: a systematic review. JAMA. 2010;304:452–460. - PubMed
-
- Vitali C, Bombardieri S, Jonsson R, Moutsopoulos HM, Alexander EL, Carsons SE, Daniels TE, Fox PC, Fox RI, Kassan SS, et al. Classification criteria for Sjögren’s syndrome: a revised version of the European criteria proposed by the American-European Consensus Group. Ann Rheum Dis. 2002;61:554–558. - PMC - PubMed
-
- Liew MS, Zhang M, Kim E, Akpek EK. Prevalence and predictors of Sjogren’s syndrome in a prospective cohort of patients with aqueous-deficient dry eye. Br J Ophthalmol. 2012;96:1498–1503. - PubMed
-
- Sall KN, Cohen SM, Christensen MT, Stein JM. An evaluation of the efficacy of a cyclosporine-based dry eye therapy when used with marketed artificial tears as supportive therapy in dry eye. Eye Contact Lens. 2006;32:21–26. - PubMed
Publication types
MeSH terms
Substances
Associated data
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Miscellaneous

