Effects of Cannabis sativa extract on haloperidol-induced catalepsy and oxidative stress in the mice
- PMID: 27366134
- PMCID: PMC4928014
Effects of Cannabis sativa extract on haloperidol-induced catalepsy and oxidative stress in the mice
Abstract
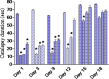
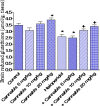
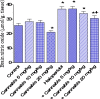
Haloperidol is a classic antipsychotic drug known for its propensity to cause extrapyramidal symptoms due to blockade of dopamine D2 receptors in the striatum. Interest in medicinal uses of cannabis is growing. Cannabis sativa has been suggested as a possible adjunctive in treatment of Parkinson's disease. The present study aimed to investigate the effect of repeated administration of an extract of Cannabis sativa on catalepsy and brain oxidative stress induced by haloperidol administration in mice. Cannabis extract was given by subcutaneous route at 5, 10 or 20 mg/kg (expressed as Δ(9)-tetrahydrocannabinol) once daily for 18 days and the effect on haloperidol (1 mg/kg, i.p.)-induced catalepsy was examined at selected time intervals using the bar test. Mice were euthanized 18 days after starting cannabis injection when biochemical assays were carried out. Malondialdehyde (MDA), reduced glutathione (GSH) and nitric oxide (the concentrations of nitrite/nitrate) were determined in brain and liver. In saline-treated mice, no catalepsy was observed at doses of cannabis up to 20 mg/kg. Mice treated with haloperidol at the dose of 1 mg/kg, exhibited significant cataleptic response. Mice treated with cannabis and haloperidol showed significant decrease in catalepsy duration, compared with the haloperidol only treated group. This decrease in catalepsy duration was evident on days 1-12 after starting cannabis injection. Later the effect of cannabis was not apparent. The administration of only cannabis (10 or 20 mg/kg) decreased brain MDA by 17.5 and 21.8 %, respectively. The level of nitric oxide decreased by 18 % after cannabis at 20 mg/kg. Glucose in brain decreased by 20.1 % after 20 mg/kg of cannabis extract. The administration of only haloperidol increased MDA (22.2 %), decreased GSH (25.7 %) and increased brain nitric oxide by 44.1 %. The administration of cannabis (10 or 20 mg/kg) to haloperidol-treated mice resulted in a significant decrease in brain MDA and nitric oxide as well as a significant increase in GSH and glucose compared with the haloperidol-control group. Cannabis had no significant effects on liver MDA, GSH, nitric oxide in saline or haloperidol-treated mice. It is concluded that cannabis improves catalepsy induced by haloperidol though the effect is not maintained on repeated cannabis administration. Cannabis alters the oxidative status of the brain in favor of reducing lipid peroxidation, but reduces brain glucose, which would impair brain energetics.
Keywords: Cannabis sativa extract; catalepsy; haloperidol; mice.
Figures




References
-
- Ashton CH. Pharmacology and effects of cannabis: A brief review. Br J Psychiat. 2001;178:101–6. - PubMed
-
- Bhattacharyya S, Fusar-Poli P, Borgwardt S, Martin-Santos R, Nosarti C, O'Carroll C, et al. Modulation of mediotemporal and ventrostriatal function in humans by Delta9-tetrahydrocannabinol: a neural basis for the effects of Cannabis sativa on learning and psychosis. Arch Gen Psychiatry. 2009;66:442–451. - PubMed
-
- Bossong MG, van Berckel BN, Boellaard R, Zuurman L, Schuit RC, Windhorst AD, et al. Delta 9-tetrahydrocannabinol induces dopamine release in the human striatum. Neuropsychopharmacology. 2009;34:759–766. - PubMed
-
- Buccellato E, Carretta D, Utan A, Cavina C, Speroni E, Grassi G, et al. Acute and chronic cannabinoid extracts administration affects motor function in a CREAE model of multiple sclerosis. J Ethnopharmacol. 2011;133:1033–1038. - PubMed
LinkOut - more resources
Full Text Sources
