Fosfomycin: A First-Line Oral Therapy for Acute Uncomplicated Cystitis
- PMID: 27366158
- PMCID: PMC4904571
- DOI: 10.1155/2016/2082693
Fosfomycin: A First-Line Oral Therapy for Acute Uncomplicated Cystitis
Abstract
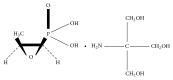
Fosfomycin is a new agent to Canada approved for the treatment of acute uncomplicated cystitis (AUC) in adult women infected with susceptible isolates of E. coli and Enterococcus faecalis. We reviewed the literature regarding the use of oral fosfomycin for the treatment of AUC. All English-language references from 1975 to October 2015 were reviewed. In Canada, fosfomycin tromethamine is manufactured as Monurol® and is available as a 3-gram single dose sachet. Fosfomycin has a unique chemical structure, inhibiting peptidoglycan synthesis at an earlier site compared to β-lactams with no cross-resistance with other agents. Fosfomycin displays broad-spectrum activity against ESBL-producing, AmpC-producing, carbapenem-non-susceptible, and multidrug-resistant (MDR) E. coli. Resistance to fosfomycin in E. coli is rare (<1%). Fosfomycin is excreted unchanged in the urine by glomerular filtration with peak urinary concentration ~4000 µg/mL and remains at concentrations >100 µg/mL for 48 hours after a single 3-gram oral dose. No dosage adjustments are required in elderly patients, in pregnant patients, or in either renal or hepatic impairment. Fosfomycin demonstrates a favorable safety profile, and clinical trials have demonstrated efficacy in AUC that is comparable to ciprofloxacin, nitrofurantoin, and trimethoprim-sulfamethoxazole. Fosfomycin's in vitro activity against common uropathogens, including MDR isolates, its favorable safety profile including pregnancy patients, drug interactions, and clinical trials data demonstrating efficacy in AUC, has resulted in Canadian, US, and European guidelines/authorities recommending fosfomycin as a first line agent for the treatment of AUC.
Figures
References
-
- Pezzlo M. A. Laboratory diagnosis of urinary tract infections: guidelines, challenges, and innovations. Clinical Microbiology Newsletter. 2014;36(12):87–93. doi: 10.1016/j.clinmicnews.2014.05.003. - DOI
-
- Mehnert-Kay S. A. Diagnosis and management of uncomplicated urinary tract infections. American Family Physician. 2005;72(3):451–458. - PubMed
Publication types
LinkOut - more resources
Full Text Sources
Other Literature Sources