Vascular Protective Role of Samul-Tang in HUVECs: Involvement of Nrf2/HO-1 and NO
- PMID: 27366195
- PMCID: PMC4913014
- DOI: 10.1155/2016/9580234
Vascular Protective Role of Samul-Tang in HUVECs: Involvement of Nrf2/HO-1 and NO
Abstract
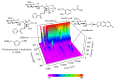
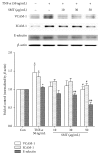
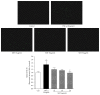
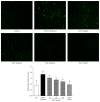
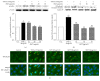
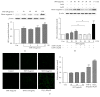
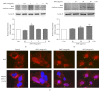
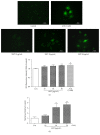
Samul-Tang (Si-Wu-Tang, SMT), composed of four medicinal herbs, is a well-known herbal formula treating hematological disorder or gynecologic disease. However, vascular protective effects of SMT and its molecular mechanisms on the vascular endothelium, known as the central spot of vascular inflammatory process, are not reported. The aim of this study was to investigate vascular protective effects of SMT water extract in human umbilical vein endothelial cells (HUVECs). Water extract of SMT was prepared and identified by HPLC-PDA analysis. Expression of cell adhesion molecules (CAMs) and heme oxygenase-1 (HO-1) and translocation of nuclear factor-kappa B (NF-κB) and nuclear factor-erythroid 2-related factor 2 (Nrf2) were determined by western blot. Nuclear localization of NF-κB and Nrf2 was visualized by immunofluorescence and DNA binding activity of NF-κB was measured. ROS production, HL-60 monocyte adhesion, and intracellular nitric oxide (NO) were also measured using a fluorescent indicator. SMT suppressed NF-κB translocation and activation as well as expression of CAMs, monocyte adhesion, and ROS production induced by TNF-α in HUVECs. SMT treated HUVECs showed upregulation of HO-1 and NO which are responsible for vascular protective action. Our study suggests that SMT, a traditionally used herbal formula, protects the vascular endothelium from inflammation and might be used as a promising vascular protective drug.
Figures

Similar articles
-
Ligustilide attenuates vascular inflammation and activates Nrf2/HO-1 induction and, NO synthesis in HUVECs.Phytomedicine. 2018 Jan 1;38:12-23. doi: 10.1016/j.phymed.2017.09.022. Epub 2017 Oct 4. Phytomedicine. 2018. PMID: 29425644
-
Nepeta angustifolia attenuates responses to vascular inflammation in high glucose-induced human umbilical vein endothelial cells through heme oxygenase-1 induction.J Ethnopharmacol. 2019 Mar 1;231:187-196. doi: 10.1016/j.jep.2018.11.015. Epub 2018 Nov 10. J Ethnopharmacol. 2019. PMID: 30419276
-
Involvement of heme oxygenase-1 induction in anti-vascular inflammation effects of Xanthoceras sorbifolia in human umbilical vein endothelial cells.J Tradit Chin Med. 2018 Dec;38(6):803-814. J Tradit Chin Med. 2018. PMID: 32186127
-
(-)-7(S)-hydroxymatairesinol protects against tumor necrosis factor-α-mediated inflammation response in endothelial cells by blocking the MAPK/NF-κB and activating Nrf2/HO-1.Phytomedicine. 2017 Aug 15;32:15-23. doi: 10.1016/j.phymed.2017.04.005. Epub 2017 Apr 10. Phytomedicine. 2017. PMID: 28732803
-
Postprandial lipoproteins and the molecular regulation of vascular homeostasis.Prog Lipid Res. 2013 Oct;52(4):446-64. doi: 10.1016/j.plipres.2013.06.001. Epub 2013 Jun 15. Prog Lipid Res. 2013. PMID: 23774609 Review.
Cited by
-
Si-Wu-Tang Alleviates Nonalcoholic Fatty Liver Disease via Blocking TLR4-JNK and Caspase-8-GSDMD Signaling Pathways.Evid Based Complement Alternat Med. 2020 Aug 11;2020:8786424. doi: 10.1155/2020/8786424. eCollection 2020. Evid Based Complement Alternat Med. 2020. PMID: 32849904 Free PMC article.
-
Activation of Nrf2/HO-1 signaling: An important molecular mechanism of herbal medicine in the treatment of atherosclerosis via the protection of vascular endothelial cells from oxidative stress.J Adv Res. 2021 Jul 6;34:43-63. doi: 10.1016/j.jare.2021.06.023. eCollection 2021 Dec. J Adv Res. 2021. PMID: 35024180 Free PMC article. Review.
-
Samul-Tang Regulates Cell Cycle and Migration of Vascular Smooth Muscle Cells against TNF-α Stimulation.Evid Based Complement Alternat Med. 2018 Jun 25;2018:1024974. doi: 10.1155/2018/1024974. eCollection 2018. Evid Based Complement Alternat Med. 2018. PMID: 30046331 Free PMC article.
-
Anti-Hypertensive Activity of Novel Peptides Identified from Olive Flounder (Paralichthys olivaceus) Surimi.Foods. 2020 May 18;9(5):647. doi: 10.3390/foods9050647. Foods. 2020. PMID: 32443419 Free PMC article.
-
Immuno-protective impact and clinical translation of radioprotective agents in cancer radiotherapy.Front Immunol. 2025 Jul 4;16:1610296. doi: 10.3389/fimmu.2025.1610296. eCollection 2025. Front Immunol. 2025. PMID: 40688089 Free PMC article. Review.
References
LinkOut - more resources
Full Text Sources
Other Literature Sources

