Cost-effectiveness analysis of rifaximin-α administration for the reduction of episodes of overt hepatic encephalopathy in recurrence compared with standard treatment in France
- PMID: 27366216
- PMCID: PMC4913340
- DOI: 10.1177/1756283X16644249
Cost-effectiveness analysis of rifaximin-α administration for the reduction of episodes of overt hepatic encephalopathy in recurrence compared with standard treatment in France
Abstract
Background: Hepatic encephalopathy (HE) is a complex neuropsychiatric syndrome that occurs most often in a context of acute or chronic liver disease. Despite the seriousness of the pathology, only a few treatments have been developed for improving its management. Rifaximin-α is the first treatment that has been clinically developed for overt HE (OHE) episodes. Recent results of clinical studies demonstrated its significant improvement in the health-related quality of life. The objective of the current study was to estimate the long-term cost-effectiveness of rifaximin-α used in combination with lactulose compared with lactulose monotherapy in cirrhotic patients, who have experienced at least two prior OHE events.
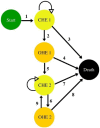
Methods: A Markov model was used to estimate rifaximin-α cost-effectiveness, evaluating it from the perspective of all contributors as recommended by French health technology assessment guidelines. Costs were based on current French treatment practices. The transition between health states was based on the reanalysis of the rifaximin-α pivotal clinical trials RFHE3001 and RFHE3002. The main outcome of the model was cost per quality adjusted life year (QALY).
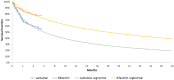
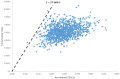
Results: The results indicate that rifaximin-α is a cost-effective treatment option with an incremental cost per QALY gained of €19,187 and €18,517 over two different time horizons (2 and 5 years). The robustness of the model was studied using probabilistic sensitivity analysis.
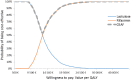
Conclusion: For the societal willingness to pay threshold of €27,000 per QALY gained, rifaximin-α in combination with lactulose is a cost-effective and affordable treatment for patients who have experienced at least two prior overt HE episodes.
Keywords: Markov chains; cost–benefit analysis; hepatic encephalopathy; lactulose; rifaximin.
Conflict of interest statement
Figures

References
-
- Bass N., Mullen K., Sanyal A., Poordad F., Neff G., Leevy C., et al. (2010) Rifaximin treatment in hepatic encephalopathy. N Engl J Med 362: 1071–1081. - PubMed
-
- Briggs A., Goeree R., Blackhouse G., O’Brien B. (2002) Probabilistic analysis of cost-effectiveness models: choosing between treatment strategies for gastroesophageal reflux disease. Med Decis Mak 22: 290–308. - PubMed
-
- Briggs A., Sculpher M., Claxton K. (2006) Decision Modelling for Health Economic Evaluation, 1st edn Oxford: OUP.
-
- Chautant F. (2013) Evaluation de l’utilisation de la rifaximine dans la prise en charge de l’encéphalopathie hépatique du patient cirrhotique au CHU de Toulouse. Thesis, Université de Limoges; Available at: http://aurore.unilim.fr/theses/nxfile/default/53adaa5a-261f-4438-8152-4c...
-
- Claxton K., Martin S., Soares M., Rice N., Spackman E., Hinde S., et al. (2013) Methods for the estimation of the NICE cost effectiveness threshold. Report, University of York, Centre for Health Economics; Available at: https://www.york.ac.uk/media/che/documents/reports/resubmitted_report.pdf
LinkOut - more resources
Full Text Sources
Other Literature Sources

