Preparation and Comprehensive Characterization of a Calcium Hydroxyapatite Reference Material
- PMID: 27366634
- PMCID: PMC4856200
- DOI: 10.6028/jres.109.042
Preparation and Comprehensive Characterization of a Calcium Hydroxyapatite Reference Material
Abstract
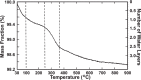
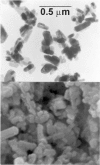
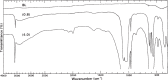
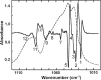
Numerous biological and chemical studies involve the use of calcium hydroxyapatite (HA), Ca10(PO4)6(OH)2. In this study detailed physicochemical characterization of HA, prepared from an aqueous solution, was carried out employing different methods and techniques: chemical and thermal analyses, x-ray diffraction, infrared and Raman spectroscopies, scanning and transmission microscopies, and Brunauer, Emmett, and Teller (BET) surface-area method. The contents of calcium (Ca(2+)), phosphate (PO4 (3-)), hydroxide (OH(-)), hydrogenphosphate (HPO4 (2-)), water (H2O), carbonate (CO3 (2-)), and trace constituents, the Ca/P molar ratio, crystal size and morphology, surface area, unit-cell parameters, crystallinity, and solubility of this HA were determined. This highly pure, homogeneous, and highly crystalline HA is certified as a National Institute of Standards and Technology (NIST) standard reference material, SRM 2910.
Keywords: Raman; chemical analysis; crystal size; crystallinity; hydroxyapatite; infrared; morphology; preparation; solubility; surface area; thermal analysis; unit-cell parameters; x-ray diffraction.
Figures


References
-
- LeGeros RZ. Calcium Phosphates in Oral Biology. Karger; Basel: 1991. - PubMed
-
- Elliott JC. Structure and Chemistry of the Apatites and Other Calcium Orthophosphates. Elsevier; Amsterdam: 1994.
-
- Arends J, Christoffersen J, Christoffersen MR, Eckert H, Fowler BO, Heughebaert JC, Nancollas GH, Yesinowski JP, Zawacki SJ. A Calcium Hydroxyapatite Precipitated from an Aqueous Solution. An International Multimethod Analysis. J Crystal Growth. 1987;84:515–532.
-
- Markovic M, Fowler BO, Tung MS, Lagergren ES. Composition and Solubility Product of a Synthetic Calcium Hydroxyapatite. Chemical and Thermal Determination of Ca/P Ratio and Statistical Analysis of Chemical and Solubility Data. In: Amjad Z, editor. Mineral and Scale Formation, Proc of ACS Symposium. Plenum; New York: 1995. pp. 271–282.
-
- Certificate of Analysis, Standard Reference Material 2910. National Institute of Standards and Technology; Gaithersburg, MD, USA: 1997.
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Miscellaneous
