In vitro evaluation of antifungal activity of monolaurin against Candida albicans biofilms
- PMID: 27366648
- PMCID: PMC4924139
- DOI: 10.7717/peerj.2148
In vitro evaluation of antifungal activity of monolaurin against Candida albicans biofilms
Abstract
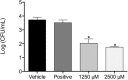
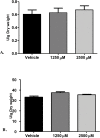
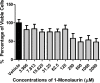
Monolaurin (also known as glycerol monolaurate) is a natural compound found in coconut oil and is known for its protective biological activities as an antimicrobial agent. The nature of oral candidiasis and the increased antifungal resistance demand the search for novel antifungal therapeutic agents. In this study, we examine the antifungal activity of monolaurin against Candida albicans biofilms (strain ATCC:SC5314/MYA2876) in vitro and investigate whether monolaurin can alter gene expression of host inflammatory cytokines, IL-1α and IL-1β. In a co-culture model, oral fibroblast cells were cultured simultaneously with C. albicans for 24 hrs followed by the exposure to treatments of monolaurin (3.9-2,500 µM), positive control fluconazole (32.2 µM), and vehicle control group (1% ethanol), which was a model used to evaluate the cytotoxicity of monolaurin on fibroblasts as well as to analyze morphological characteristics of biofilms through fluorescence microscopy. In addition, the co-culture model was used for RNA extraction of oral fibroblasts to assess gene expression of host inflammatory cytokines, using quantitative real-time PCR. Our results showed the MIC and MFC of monolaurin were in the range 62.5-125 µM and 125-250 µM, respectively. Biofilm antifungal assay showed significant reduction in Log (CFU/ml) of biofilms treated with 1,250 and 2,500 µM of 1-monolaurin when compared to the control groups . There was also a significant down-regulation of IL-1α and IL-1β in the co-culture treated with monolaurin. It can be concluded that monolaurin has a potential antifungal activity against C. albicans and can modulate the pro-inflammatory response of the host.
Keywords: Antimicrobial agent; Biofilms; Candida albicans; Host inflammatory response; In vitro; MIC/MFC; Monolaurin; Oral candidiasis; Proteolytic enzymes; Virulence factors.
Conflict of interest statement
The authors declare there are no competing interests.
Figures


Similar articles
-
Fungal-Host Interaction: Curcumin Modulates Proteolytic Enzyme Activity of Candida albicans and Inflammatory Host Response In Vitro.Int J Dent. 2018 Aug 15;2018:2393146. doi: 10.1155/2018/2393146. eCollection 2018. Int J Dent. 2018. PMID: 30186325 Free PMC article.
-
In Vivo Antifungal Activity of Monolaurin against Candida albicans Biofilms.Biol Pharm Bull. 2018;41(8):1299-1302. doi: 10.1248/bpb.b18-00256. Biol Pharm Bull. 2018. PMID: 30068882
-
Yeast-Host Interactions: Anadenanthera colubrina Modulates Virulence Factors of C. albicans and Inflammatory Response In Vitro.Front Pharmacol. 2021 Jun 8;12:629778. doi: 10.3389/fphar.2021.629778. eCollection 2021. Front Pharmacol. 2021. PMID: 34168555 Free PMC article.
-
In Vitro and In Vivo Antifungal Activity of Lichochalcone-A against Candida albicans Biofilms.PLoS One. 2016 Jun 10;11(6):e0157188. doi: 10.1371/journal.pone.0157188. eCollection 2016. PLoS One. 2016. PMID: 27284694 Free PMC article.
-
Thymus vulgaris essential oil and thymol inhibit biofilms and interact synergistically with antifungal drugs against drug resistant strains of Candida albicans and Candida tropicalis.J Mycol Med. 2020 Apr;30(1):100911. doi: 10.1016/j.mycmed.2019.100911. Epub 2019 Nov 7. J Mycol Med. 2020. PMID: 32008964
Cited by
-
Fungal-Host Interaction: Curcumin Modulates Proteolytic Enzyme Activity of Candida albicans and Inflammatory Host Response In Vitro.Int J Dent. 2018 Aug 15;2018:2393146. doi: 10.1155/2018/2393146. eCollection 2018. Int J Dent. 2018. PMID: 30186325 Free PMC article.
-
Bioactive Dental Adhesive System With tt-Farnesol: Effects on Dental Biofilm and Bonding Properties.Front Bioeng Biotechnol. 2020 Jul 23;8:865. doi: 10.3389/fbioe.2020.00865. eCollection 2020. Front Bioeng Biotechnol. 2020. PMID: 32793584 Free PMC article.
-
Antifungal Resistance, Metabolic Routes as Drug Targets, and New Antifungal Agents: An Overview about Endemic Dimorphic Fungi.Mediators Inflamm. 2017;2017:9870679. doi: 10.1155/2017/9870679. Epub 2017 Jun 13. Mediators Inflamm. 2017. PMID: 28694566 Free PMC article. Review.
-
Ascorbic Acid Enhances the Inhibitory Effect of Theasaponins against Candida albicans.Int J Mol Sci. 2024 Oct 3;25(19):10661. doi: 10.3390/ijms251910661. Int J Mol Sci. 2024. PMID: 39408989 Free PMC article.
-
Healthy Diet and Lifestyle Improve the Gut Microbiota and Help Combat Fungal Infection.Microorganisms. 2023 Jun 11;11(6):1556. doi: 10.3390/microorganisms11061556. Microorganisms. 2023. PMID: 37375058 Free PMC article. Review.
References
-
- Arien KK, Vanham G, Gali Y. A dual-chamber model ofthe female genital tract to evaluate epithelial toxicity of candidate anti-HIV microbicides. Current Protocols in Cell Biology, Chapter. 2011;26(Unit26):13. - PubMed
-
- Carpo BG, Verallo-Rowell VM, Kabara J. Novel antibacterial activity of monolaurin compared with conventionalantibiotics against organisms from skin infections: an in vitro study. Journal of Drugs in Dermatology. 2007;6(10):991–998. - PubMed
-
- Chandra J, McCormick TS, Imamura Y, Mukherjee PK, Ghannoum MA. Interaction of Candida albicans with adherent human peripheral blood mononuclear cells increases C. albicans biofilm formation and results in differential expression of pro- and anti-inflammatory cytokines. Infection and Immunity. 2007;75(5):2612–2620. doi: 10.1128/IAI.01841-06. - DOI - PMC - PubMed
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources

