Organoarsenical Biotransformations by Shewanella putrefaciens
- PMID: 27366920
- PMCID: PMC4984541
- DOI: 10.1021/acs.est.6b00235
Organoarsenical Biotransformations by Shewanella putrefaciens
Abstract
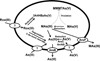
Microbes play a critical role in the global arsenic biogeocycle. Most studies have focused on redox cycling of inorganic arsenic in bacteria and archaea. The parallel cycles of organoarsenical biotransformations are less well characterized. Here we describe organoarsenical biotransformations in the environmental microbe Shewanella putrefaciens. Under aerobic growth conditions, S. putrefaciens reduced the herbicide MSMA (methylarsenate or MAs(V)) to methylarsenite (MAs(III)). Even though it does not contain an arsI gene, which encodes the ArsI C-As lyase, S. putrefaciens demethylated MAs(III) to As(III). It cleaved the C-As bond in aromatic arsenicals such as the trivalent forms of the antimicrobial agents roxarsone (Rox(III)), nitarsone (Nit(III)) and phenylarsenite (PhAs(III)), which have been used as growth promoters for poultry and swine. S. putrefaciens thiolated methylated arsenicals, converting MAs(V) into the more toxic metabolite monomethyl monothioarsenate (MMMTAs(V)), and transformed dimethylarsenate (DMAs(V)) into dimethylmonothioarsenate (DMMTAs(V)). It also reduced the nitro groups of Nit(V), forming p-aminophenyl arsenate (p-arsanilic acid or p-AsA(V)), and Rox(III), forming 3-amino-4-hydroxybenzylarsonate (3A4HBzAs(V)). Elucidation of organoarsenical biotransformations by S. putrefaciens provides a holistic appreciation of how these environmental pollutants are degraded.
Figures


References
-
- Li Y, Liu Z. Arsenic toxicity: toxicity, manifestation and geographical distribution. In: Chakrabarty N, editor. Arsenic Toxicity: Prevention and Treatment. Boca Raton, FL: CRC Press; 2016. pp. 45–78.
-
- Quinn JP, McMullan G. Carbon-arsenic bond cleavage by a newly isolated gram-negative bacterium, strain ASV2. Microbiology. 1995;141(Pt 3):721–725. - PubMed
-
- Naranmandura H, Rehman K, Le XC, Thomas DJ. Formation of methylated oxyarsenicals and thioarsenicals in wild-type and arsenic (+3 oxidation state) methyltransferase knockout mice exposed to arsenate. Anal. Bioanal. Chem. 2013;405(6):1885–1891. - PubMed
-
- Wang QQ, Thomas DJ, Naranmandura H. Importance of being thiomethylated: formation, fate, and effects of methylated thioarsenicals. Chem. Res. Toxicol. 2015;28(3):281–289. - PubMed
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Research Materials

