Multifunctional near-infrared light-triggered biodegradable micelles for chemo- and photo-thermal combination therapy
- PMID: 27366951
- PMCID: PMC5347683
- DOI: 10.18632/oncotarget.10320
Multifunctional near-infrared light-triggered biodegradable micelles for chemo- and photo-thermal combination therapy
Abstract
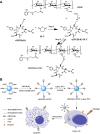
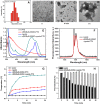
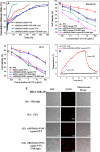
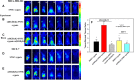
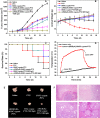
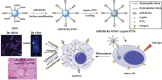
A combination of chemo- and photo-thermal therapy (PTT) has provided a promising efficient approach for cancer therapy. To achieve the superior synergistic chemotherapeutic effect with PTT, the development of a simple theranostic nanoplatform that can provide both cancer imaging and a spatial-temporal synchronism of both therapeutic approaches are highly desired. Our previous study has demonstrated that near-infrared (NIR) light-triggered biodegradable chitosan-based amphiphilic block copolymer micelles (SNSC) containing light-sensitive 2-nitrobenzyl alcohol and NIR dye cypate on the hydrophobic block could be used for fast light-triggered drug release. In this study, we conjugated the SNSC micelles with tumor targeting ligand c(RGDyK) and also encapsulated antitumor drug Paclitaxel (PTX). The results show that c(RGDyK)-modified micelles could enhance the targeting and residence time in tumor site, as well as be capable performing high temperature response for PTT on cancer cells and two-photon photolysis for fast release of anticancer drugs under NIR irradiation. In vitro release profiles show a significant controlled release effort that the release concentration of PTX from micelles was significantly increased with the exposure of NIR light. In vitro and in vivo antitumor studies demonstrate that, compared with chemo or PTT treatment alone, the combined treatment with the local exposure of NIR light exhibited significantly enhanced anti-tumor efficiency. These findings indicate that this system exhibited great potential in tumor-targeting imaging and synchronous chemo- and photo-thermal therapy.
Keywords: chemotherapy; near-infrared light-triggered nanomicelles; paclitaxel; photo-thermal therapy; tumor targeting.
Conflict of interest statement
The authors declare that no conflicts of interest exists in the present study.
Figures

Similar articles
-
Near-infrared light-triggered micelles for fast controlled drug release in deep tissue.Biomaterials. 2013 Aug;34(26):6272-83. doi: 10.1016/j.biomaterials.2013.05.008. Epub 2013 May 27. Biomaterials. 2013. PMID: 23721796
-
Cyclic RGD conjugated poly(ethylene glycol)-co-poly(lactic acid) micelle enhances paclitaxel anti-glioblastoma effect.J Control Release. 2010 Apr 2;143(1):136-42. doi: 10.1016/j.jconrel.2009.12.020. Epub 2010 Jan 7. J Control Release. 2010. PMID: 20056123
-
Near-infrared light triggered drug delivery system for higher efficacy of combined chemo-photothermal treatment.Acta Biomater. 2017 Mar 15;51:374-392. doi: 10.1016/j.actbio.2016.12.004. Epub 2017 Jan 11. Acta Biomater. 2017. PMID: 28088668
-
In vitro outlook of gold nanoparticles in photo-thermal therapy: a literature review.Lasers Med Sci. 2018 May;33(4):917-926. doi: 10.1007/s10103-018-2467-z. Epub 2018 Feb 28. Lasers Med Sci. 2018. PMID: 29492712 Review.
-
Near-infrared photoresponsive drug delivery nanosystems for cancer photo-chemotherapy.J Nanobiotechnology. 2020 Aug 3;18(1):108. doi: 10.1186/s12951-020-00668-5. J Nanobiotechnology. 2020. PMID: 32746846 Free PMC article. Review.
Cited by
-
Magnetic Nano-Platform Enhanced iPSC-Derived Trabecular Meshwork Delivery and Tracking Efficiency.Int J Nanomedicine. 2022 Mar 22;17:1285-1307. doi: 10.2147/IJN.S346141. eCollection 2022. Int J Nanomedicine. 2022. PMID: 35345785 Free PMC article.
-
Stimuli-Responsive Polymeric Nanoplatforms for Cancer Therapy.Front Bioeng Biotechnol. 2021 Jun 25;9:707319. doi: 10.3389/fbioe.2021.707319. eCollection 2021. Front Bioeng Biotechnol. 2021. PMID: 34249894 Free PMC article. Review.
-
Micellization of Photo-Responsive Block Copolymers.Polymers (Basel). 2017 Aug 26;9(9):396. doi: 10.3390/polym9090396. Polymers (Basel). 2017. PMID: 30965699 Free PMC article. Review.
-
Recent advances in light-responsive on-demand drug-delivery systems.Ther Deliv. 2017 Feb;8(2):89-107. doi: 10.4155/tde-2016-0060. Ther Deliv. 2017. PMID: 28088880 Free PMC article. Review.
-
Hyaluronic Acid Layer-By-Layer (LbL) Nanoparticles for Synergistic Chemo-Phototherapy.Pharm Res. 2018 Aug 24;35(10):196. doi: 10.1007/s11095-018-2480-8. Pharm Res. 2018. PMID: 30143878
References
-
- Gao J, Zhang W, Huang P, Zhang B, Zhang X, Xu B. Intracellular spatial control of fluorescent magnetic nanoparticles. J. Am. Chem. Soc. 2008;130:3710–3711. - PubMed
-
- Yu MK, Jeong YY, Park J, Park S, Kim JW, Min JJ, Kim K, Jon S. Drug-loaded superparamagnetic iron oxide nanoparticles for combined cancer imaging and therapy in vivo. Angew. Chem. Int. Ed. 2008;47:5362–5365. - PubMed
-
- Lane D. Design combination therapy for cancer. Nat. Biotechnol. 2006;24:163–164. - PubMed
-
- Alivisatos AP. The use of nanocrystals in biological detection. Nat. Biotechnol. 2004;22:47–52. - PubMed
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Miscellaneous

