Drug disposition before and after gastric bypass: fenofibrate and posaconazole
- PMID: 27367040
- PMCID: PMC5061797
- DOI: 10.1111/bcp.13054
Drug disposition before and after gastric bypass: fenofibrate and posaconazole
Abstract
Aims: Roux-en-Y gastric bypass (RYGB) alters the anatomical structure of the gastrointestinal tract, which can result in alterations in drug disposition. The aim of the present study was to evaluate the oral disposition of two compounds belonging to the Biopharmaceutical Classification System Class II - fenofibrate (bile salt-dependent solubility) and posaconazole (gastric pH-dependent dissolution) - before and after RYGB in the same individuals.
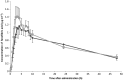
Methods: A single-dose pharmacokinetic study with two model compounds - namely, 67 mg fenofibrate (Lipanthyl®) and 400 mg posaconazole (Noxafil®) - was performed in 12 volunteers pre- and post-RYGB. After oral administration, blood samples were collected at different time points up to 48 h after administration. Plasma concentrations were determined by high-performance liquid chromatography in order to calculate the area under the concentration-time curve up to 48 h (AUC0-48 h ), the peak plasma concentration (Cmax) and the time to reach peak concentration (Tmax ).
Results: After administration of fenofibrate, no relevant differences in AUC0-48 h , Cmax and Tmax between the pre- and postoperative setting were observed. The geometric mean of the ratio of AUC0-48 h post/pre-RYGB for fenofibrate was 1.10 [95% confidence interval (CI) 0.87, 1.40; P = 0.40]. For posaconazole, an important decrease in AUC0-48 h and Cmax following RYGB was shown; the geometric mean of the AUC0-48 h post/pre-RYGB ratio was 0.68 (95% CI 0.48, 0.96; P = 0.03) and the geometric mean of the Cmax pre/post-RYGB ratio was 0.60 (95% CI 0.39, 0.94; P = 0.03). The decreased exposure of posaconazole could be explained by the increased gastric pH and accelerated gastric emptying of fluids post-RYGB. No difference for Tmax was observed.
Conclusions: The disposition of fenofibrate was not altered after RYGB, whereas the oral disposition of posaconazole was significantly decreased following RYGB.
Keywords: Roux-en-Y gastric bypass; absorption; bariatric surgery; fenofibrate; pharmacokinetics; posaconazole.
© 2016 The British Pharmacological Society.
Figures
 Preoperative,
Preoperative,  Postoperative
Postoperative
 Preoperative,
Preoperative,  Postoperative
PostoperativeSimilar articles
-
Predicting iron absorption from an effervescent iron supplement in obese patients before and after Roux-en-Y gastric bypass: a preliminary study.J Trace Elem Med Biol. 2019 Mar;52:68-73. doi: 10.1016/j.jtemb.2018.12.002. Epub 2018 Dec 7. J Trace Elem Med Biol. 2019. PMID: 30732902
-
PBPK modeling of CYP3A and P-gp substrates to predict drug-drug interactions in patients undergoing Roux-en-Y gastric bypass surgery.J Pharmacokinet Pharmacodyn. 2020 Oct;47(5):493-512. doi: 10.1007/s10928-020-09701-4. Epub 2020 Jul 24. J Pharmacokinet Pharmacodyn. 2020. PMID: 32710209
-
Pharmacokinetics of oral levonorgestrel and ethinylestradiol in women after Roux-en-Y gastric bypass surgery.Surg Obes Relat Dis. 2021 Apr;17(4):673-681. doi: 10.1016/j.soard.2020.12.007. Epub 2020 Dec 16. Surg Obes Relat Dis. 2021. PMID: 33547015
-
RYGB and Drug Disposition: How to Do Better? Analysis of Pharmacokinetic Studies and Recommendations for Clinical Practice.Obes Surg. 2017 Apr;27(4):1076-1090. doi: 10.1007/s11695-016-2535-z. Obes Surg. 2017. PMID: 28124236 Review.
-
Iron and Vitamin D/Calcium Deficiency after Gastric Bypass: Mechanisms Involved and Strategies to Improve Oral Supplement Disposition.Curr Drug Metab. 2019;20(3):244-252. doi: 10.2174/1389200219666181026160242. Curr Drug Metab. 2019. PMID: 30362417 Review.
Cited by
-
The Effects of Bariatric Surgery on Pharmacokinetics of Drugs: a Review of Current Evidence.Curr Nutr Rep. 2023 Dec;12(4):695-708. doi: 10.1007/s13668-023-00498-5. Epub 2023 Oct 20. Curr Nutr Rep. 2023. PMID: 37857987 Free PMC article. Review.
-
The influence of bariatric surgery on oral drug bioavailability in patients with obesity: A systematic review.Obes Rev. 2019 Sep;20(9):1299-1311. doi: 10.1111/obr.12869. Epub 2019 Jun 24. Obes Rev. 2019. PMID: 31232513 Free PMC article.
-
Oral drug dosing following bariatric surgery: General concepts and specific dosing advice.Br J Clin Pharmacol. 2021 Dec;87(12):4560-4576. doi: 10.1111/bcp.14913. Epub 2021 Jun 3. Br J Clin Pharmacol. 2021. PMID: 33990981 Free PMC article. Review.
-
Changes in Antidepressant Absorption After Metabolic Bariatric Surgery.Obes Surg. 2025 Aug;35(8):3173-3181. doi: 10.1007/s11695-025-08025-x. Epub 2025 Jul 4. Obes Surg. 2025. PMID: 40610843 Free PMC article.
-
Updated antimicrobial dosing recommendations for obese patients.Antimicrob Agents Chemother. 2024 May 2;68(5):e0171923. doi: 10.1128/aac.01719-23. Epub 2024 Mar 25. Antimicrob Agents Chemother. 2024. PMID: 38526051 Free PMC article.
References
-
- WHO – Obesity and overweight 2015. Fact sheet N°311.
-
- Picot J, Jones J, Colquitt JL, Gospodarevskaya E, Loveman E, Baxter L, et al. The clinical effectiveness and cost‐effectiveness of bariatric (weight loss) surgery for obesity: a systematic review and economic evaluation. Health Technol Assess 2009; 13: 215–357. - PubMed
-
- De Smet J, Van Boxclaer J, Boussery K. The influence of bypass procedures and other anatomical changes in the gastrointestinal tract on the oral bioavailability of drugs. J Clin Pharmacol 2013; 53: 361–76. - PubMed
-
- Padwal R, Brocks D, Sharma AM. A systematic review of drug absorption following bariatric surgery and its theoretical implications. Obes Rev 2010; 11: 41–50. - PubMed
-
- Padwal RS, Ben‐Eltriki M, Wang X, Langkaas LA, Sharma AM, Birch DW, et al. Effect of gastric bypass surgery on azithromycin oral bioavailability. J Antimicrob Chemother 2012; 67: 2203–6. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Research Materials

