MPT0B098, a Microtubule Inhibitor, Suppresses JAK2/STAT3 Signaling Pathway through Modulation of SOCS3 Stability in Oral Squamous Cell Carcinoma
- PMID: 27367272
- PMCID: PMC4930189
- DOI: 10.1371/journal.pone.0158440
MPT0B098, a Microtubule Inhibitor, Suppresses JAK2/STAT3 Signaling Pathway through Modulation of SOCS3 Stability in Oral Squamous Cell Carcinoma
Abstract
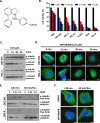
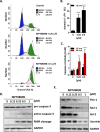
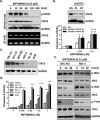
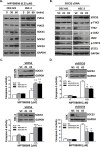
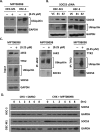
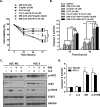
Microtubule inhibitors have been shown to inhibit Janus kinase 2/signal transducer and activator of transcription 3 (JAK2/STAT3) signal transduction pathway in various cancer cells. However, little is known of the mechanism by which the microtubule inhibitors inhibit STAT3 activity. In the present study, we examined the effect of a novel small-molecule microtubule inhibitor, MPT0B098, on STAT3 signaling in oral squamous cell carcinoma (OSCC). Treatment of various OSCC cells with MPT0B098 induced growth inhibition, cell cycle arrest and apoptosis, as well as increased the protein level of SOCS3. The accumulation of SOCS3 protein enhanced its binding to JAK2 and TYK2 which facilitated the ubiquitination and degradation of JAK2 and TYK2, resulting in a loss of STAT3 activity. The inhibition of STAT3 activity led to sensitization of OSCC cells to MPT0B098 cytotoxicity, indicating that STAT3 is a key mediator of drug resistance in oral carcinogenesis. Moreover, the combination of MPT0B098 with the clinical drug cisplatin or 5-FU significantly augmented growth inhibition and apoptosis in OSCC cells. Taken together, our results provide a novel mechanism for the action of MPT0B098 in which the JAK2/STAT3 signaling pathway is suppressed through the modulation of SOCS3 protein level. The findings also provide a promising combinational therapy of MPT0B098 for OSCC.
Conflict of interest statement
Figures
Similar articles
-
JAK2 regulation by licochalcone H inhibits the cell growth and induces apoptosis in oral squamous cell carcinoma.Phytomedicine. 2019 Jan;52:60-69. doi: 10.1016/j.phymed.2018.09.180. Epub 2018 Sep 18. Phytomedicine. 2019. PMID: 30599913
-
Licochalcone C induced apoptosis in human oral squamous cell carcinoma cells by regulation of the JAK2/STAT3 signaling pathway.J Cell Biochem. 2018 Dec;119(12):10118-10130. doi: 10.1002/jcb.27349. Epub 2018 Aug 20. J Cell Biochem. 2018. PMID: 30129052
-
MPT0B098, a novel microtubule inhibitor that destabilizes the hypoxia-inducible factor-1α mRNA through decreasing nuclear-cytoplasmic translocation of RNA-binding protein HuR.Mol Cancer Ther. 2013 Jul;12(7):1202-12. doi: 10.1158/1535-7163.MCT-12-0778. Epub 2013 Apr 25. Mol Cancer Ther. 2013. PMID: 23619299
-
The roles of SOCS3 and STAT3 in bacterial infection and inflammatory diseases.Scand J Immunol. 2018 Dec;88(6):e12727. doi: 10.1111/sji.12727. Scand J Immunol. 2018. PMID: 30341772 Review.
-
STAT3 and Its Targeting Inhibitors in Oral Squamous Cell Carcinoma.Cells. 2022 Oct 5;11(19):3131. doi: 10.3390/cells11193131. Cells. 2022. PMID: 36231093 Free PMC article. Review.
Cited by
-
Apoptosis induced by methanol extract of Potentilla discolor in human mucoepidermoid carcinoma cells through STAT3/PUMA signaling axis.Mol Med Rep. 2018 Apr;17(4):5258-5264. doi: 10.3892/mmr.2018.8468. Epub 2018 Jan 22. Mol Med Rep. 2018. PMID: 29363716 Free PMC article.
-
Blockade of the STAT3/BCL-xL Axis Leads to the Cytotoxic and Cisplatin-Sensitizing Effects of Fucoxanthin, a Marine-Derived Carotenoid, on Human Bladder Urothelial Carcinoma Cells.Mar Drugs. 2025 Jan 22;23(2):54. doi: 10.3390/md23020054. Mar Drugs. 2025. PMID: 39997178 Free PMC article.
-
CCL18-NIR1 promotes oral cancer cell growth and metastasis by activating the JAK2/STAT3 signaling pathway.BMC Cancer. 2020 Jul 8;20(1):632. doi: 10.1186/s12885-020-07073-z. BMC Cancer. 2020. PMID: 32641093 Free PMC article.
-
Lycorine exerts antitumor activity against osteosarcoma cells in vitro and in vivo xenograft model through the JAK2/STAT3 pathway.Onco Targets Ther. 2019 Jul 8;12:5377-5388. doi: 10.2147/OTT.S202026. eCollection 2019. Onco Targets Ther. 2019. PMID: 31371981 Free PMC article.
-
Novel microtubule inhibitor MPT0B098 inhibits hypoxia-induced epithelial-to-mesenchymal transition in head and neck squamous cell carcinoma.J Biomed Sci. 2018 Mar 28;25(1):28. doi: 10.1186/s12929-018-0432-6. J Biomed Sci. 2018. PMID: 29592811 Free PMC article.
References
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Research Materials
Miscellaneous

