Novel C-Ring-Hydroxy-Substituted Controlled Deactivation Cannabinergic Analogues
- PMID: 27367336
- PMCID: PMC5532543
- DOI: 10.1021/acs.jmedchem.6b00717
Novel C-Ring-Hydroxy-Substituted Controlled Deactivation Cannabinergic Analogues
Abstract
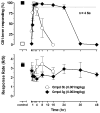
In pursuit of safer controlled-deactivation cannabinoids with high potency and short duration of action, we report the design, synthesis, and pharmacological evaluation of novel C9- and C11-hydroxy-substituted hexahydrocannabinol (HHC) and tetrahydrocannabinol (THC) analogues in which a seven atom long side chain, with or without 1'-substituents, carries a metabolically labile 2',3'-ester group. Importantly, in vivo studies validated our controlled deactivation approach in rodents and non-human primates. The lead molecule identified here, namely, butyl-2-[(6aR,9R,10aR)-1-hydroxy-9-(hydroxymethyl)-6,6-dimethyl-6a,7,8,9,10,10a-hexahydro-6H-benzo[c]chromen-3-yl]-2-methylpropanoate (AM7499), was found to exhibit remarkably high in vitro and in vivo potency with shorter duration of action than the currently existing classical cannabinoid agonists.
Conflict of interest statement
The authors declare no competing financial interest.
Figures




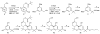



References
-
- Devane WA, Dysarz FA, 3rd, Johnson MR, Melvin LS, Howlett AC. Determination and characterization of a cannabinoid receptor in rat brain. Mol Pharmacol. 1988;34:605–613. - PubMed
-
- Munro S, Thomas KL, Abu-Shaar M. Molecular characterization of a peripheral receptor for cannabinoids. Nature. 1993;365:61–65. - PubMed
-
- Makriyannis A, Nikas SP, Thakur GA, Pavlopoulos S. Cannabinoid receptors as therapeutic targets. Curr Pharm Des. 2006;12:1751–1769. - PubMed
-
- Han S, Thatte J, Buzard DJ, Jones RM. Therapeutic utility of cannabinoid receptor type 2 (CB2) selective agonists. J Med Chem. 2013;56:8224–8256. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Chemical Information
Molecular Biology Databases
Research Materials
Miscellaneous

