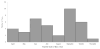Hepatotoxicity associated with weight loss or sports dietary supplements, including OxyELITE Pro™ - United States, 2013
- PMID: 27367536
- PMCID: PMC5579712
- DOI: 10.1002/dta.2036
Hepatotoxicity associated with weight loss or sports dietary supplements, including OxyELITE Pro™ - United States, 2013
Abstract
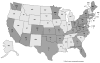
In September 2013, the Hawaii Department of Health (HDOH) was notified of seven adults who developed acute hepatitis after taking OxyELITE Pro™, a weight loss and sports dietary supplement. CDC assisted HDOH with their investigation, then conducted case-finding outside of Hawaii with FDA and the Department of Defense (DoD). We defined cases as acute hepatitis of unknown etiology that occurred from April 1, 2013, through December 5, 2013, following exposure to a weight loss or muscle-building dietary supplement, such as OxyELITE Pro™. We conducted case-finding through multiple sources, including data from poison centers (National Poison Data System [NPDS]) and FDA MedWatch. We identified 40 case-patients in 23 states and two military bases with acute hepatitis of unknown etiology and exposure to a weight loss or muscle building dietary supplement. Of 35 case-patients who reported their race, 15 (42.9%) reported white and 9 (25.7%) reported Asian. Commonly reported symptoms included jaundice, fatigue, and dark urine. Twenty-five (62.5%) case-patients reported taking OxyELITE Pro™. Of these 25 patients, 17 of 22 (77.3%) with available data were hospitalized and 1 received a liver transplant. NPDS and FDA MedWatch each captured seven (17.5%) case-patients. Improving the ability to search surveillance systems like NPDS and FDA MedWatch for individual and grouped dietary supplements, as well as coordinating case-finding with DoD, may benefit ongoing surveillance efforts and future outbreak responses involving adverse health effects from dietary supplements. This investigation highlights opportunities and challenges in using multiple sources to identify cases of suspected supplement associated adverse events. Published 2016. This article is a U.S. Government work and is in the public domain in the USA.
Keywords: OxyELITE Pro; Supplement; hepatitis.
Published 2016. This article is a U.S. Government work and is in the public domain in the USA.
Figures
References
-
- Blanck HM, Serdula MK, Gillespie C, Galuska DA, Sharpe PA, Conway JM, Khan LK, Ainsworth BE. Use of Nonprescription Dietary Supplements for Weight Loss Is Common among Americans. J Am Diet Assoc. 2007;107:441. - PubMed
-
- Navarro VJ. Herbal and dietary supplement hepatotoxicity. Semin Liver Dis. 2009;29:373. - PubMed
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Miscellaneous