Artonin E and Structural Analogs from Artocarpus Species Abrogates Estrogen Receptor Signaling in Breast Cancer
- PMID: 27367662
- PMCID: PMC6272880
- DOI: 10.3390/molecules21070839
Artonin E and Structural Analogs from Artocarpus Species Abrogates Estrogen Receptor Signaling in Breast Cancer
Abstract
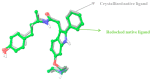
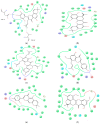
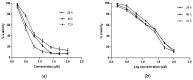
The increasing rate of mortality ensued from breast cancer has encouraged research into safer and efficient therapy. The human Estrogen receptor α has been implicated in the majority of reported breast cancer cases. Molecular docking employing Glide, Schrodinger suite 2015, was used to study the binding affinities of small molecules from the Artocarpus species after their drug-like properties were ascertained. The structure of the ligand-binding domain of human Estrogen receptor α was retrieved from Protein Data Bank while the structures of compounds were collected from PubChem database. The binding interactions of the studied compounds were reported as well as their glide scores. The best glide scored ligand, was Artonin E with a score of -12.72 Kcal when compared to other studied phytomolecules and it evoked growth inhibition of an estrogen receptor positive breast cancer cells in submicromolar concentration (3.8-6.9 µM) in comparison to a reference standard Tamoxifen (18.9-24.1 µM) within the tested time point (24-72 h). The studied ligands, which had good interactions with the target receptor, were also drug-like when compared with 95% of orally available drugs with the exception of Artoelastin, whose predicted physicochemical properties rendered it less drug-like. The in silico physicochemical properties, docking interactions and growth inhibition of the best glide scorer are indications of the anti-breast cancer relevance of the studied molecules.
Keywords: Artocarpus; Artonin E; human estrogen receptor; in silico; molecular docking.
Conflict of interest statement
The authors declare no conflict of interest.
Figures


References
-
- Nilsson S., Makela S., Treuter E., Tujague M., Thomsen J., Andersson G., Enmark E., Pettersson K., Warner M., Gustafsson J.A. Mechanism of Estrogen Action. Physiol. Rev. 2001;81:1535–1565. - PubMed
-
- Belev B., Vrbanec D. Hormonal resistance in breast- and prostate cancer. Period Biol. 2012;114:511–517.
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical

