One Pot Selective Arylation of 2-Bromo-5-Chloro Thiophene; Molecular Structure Investigation via Density Functional Theory (DFT), X-ray Analysis, and Their Biological Activities
- PMID: 27367666
- PMCID: PMC4964363
- DOI: 10.3390/ijms17070912
One Pot Selective Arylation of 2-Bromo-5-Chloro Thiophene; Molecular Structure Investigation via Density Functional Theory (DFT), X-ray Analysis, and Their Biological Activities
Abstract
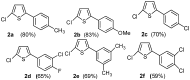
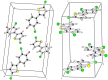
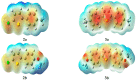
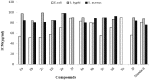
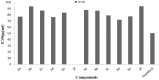
Synthesis of 2,5-bisarylthiophenes was accomplished by sequential Suzuki cross coupling reaction of 2-bromo-5-chloro thiophenes. Density functional theory (DFT) studies were carried out at the B3LYP/6-31G(d, p) level of theory to compare the geometric parameters of 2,5-bisarylthiophenes with those from X-ray diffraction results. The synthesized compounds are screened for in vitro bacteria scavenging abilities. At the concentration of 50 and 100 μg/mL, compounds 2b, 2c, 2d, 3c, and 3f with IC50-values of 51.4, 52.10, 58.0, 56.2, and 56.5 μg/mL respectively, were found most potent against E. coli. Among all the synthesized compounds 2a, 2d, 3c, and 3e with the least values of IC50 77, 76.26, 79.13 μg/mL respectively showed significant antioxidant activities. Almost all of the compounds showed good antibacterial activity against Escherichia coli, whereas 2-chloro-5-(4-methoxyphenyl) thiophene (2b) was found most active among all synthesized compound with an IC50 value of 51.4 μg/mL. All of the synthesized compounds were screened for nitric oxide scavenging activity as well. Frontier molecular orbitals (FMOs) and molecular electrostatic potentials of the target compounds were also studied theoretically to account for their relative reactivity.
Keywords: 2-bromo-5-chloro thiophenes; Suzuki coupling; antibacterial; antioxidant; density functional theory (DFT).
Figures







References
-
- Proutiere F., Aufiero M., Schoenebeck F. Reactivity and stability of dinuclear Pd(I) complexes: Studies on the active catalytic species, insights into precatalyst activation and deactivation, and application in highly selective cross-coupling reactions. J. Am. Chem. Soc. 2011;134:606–612. doi: 10.1021/ja209424z. - DOI - PubMed
-
- Joshaghani M., Daryanavard M., Rafiee E., Nadri S. Synthesis and applications of a new palladacycle as a high active catalyst in the Suzuki couplings. J. Organomet. Chem. 2008;693:3135–3140. doi: 10.1016/j.jorganchem.2008.07.003. - DOI
-
- Mora M., Jiménez-Sanchidrián C., Ruiz J.R. Suzuki cross-coupling reactions over Pd(II)-hydrotalcite catalysts in water. J. Mol. Catal. A Chem. 2008;285:79–83. doi: 10.1016/j.molcata.2008.01.031. - DOI
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical

