Mechanisms Underlying Activation of α₁-Adrenergic Receptor-Induced Trafficking of AQP5 in Rat Parotid Acinar Cells under Isotonic or Hypotonic Conditions
- PMID: 27367668
- PMCID: PMC4964398
- DOI: 10.3390/ijms17071022
Mechanisms Underlying Activation of α₁-Adrenergic Receptor-Induced Trafficking of AQP5 in Rat Parotid Acinar Cells under Isotonic or Hypotonic Conditions
Abstract
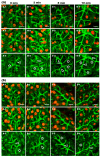
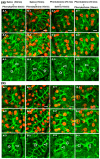
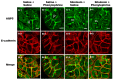
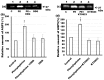
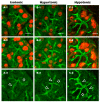
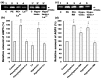
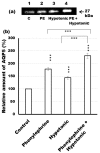
Defective cellular trafficking of aquaporin-5 (AQP5) to the apical plasma membrane (APM) in salivary glands is associated with the loss of salivary fluid secretion. To examine mechanisms of α₁-adrenoceptor (AR)-induced trafficking of AQP5, immunoconfocal microscopy and Western blot analysis were used to analyze AQP5 localization in parotid tissues stimulated with phenylephrine under different osmolality. Phenylephrine-induced trafficking of AQP5 to the APM and lateral plasma membrane (LPM) was mediated via the α1A-AR subtype, but not the α1B- and α1D-AR subtypes. Phenylephrine-induced trafficking of AQP5 was inhibited by ODQ and KT5823, inhibitors of nitric oxide (NO)-stimulated guanylcyclase (GC) and protein kinase (PK) G, respectively, indicating the involvement of the NO/ soluble (c) GC/PKG signaling pathway. Under isotonic conditions, phenylephrine-induced trafficking was inhibited by La(3+), implying the participation of store-operated Ca(2+) channel. Under hypotonic conditions, phenylephrine-induced trafficking of AQP5 to the APM was higher than that under isotonic conditions. Under non-stimulated conditions, hypotonicity-induced trafficking of AQP5 to the APM was inhibited by ruthenium red and La(3+), suggesting the involvement of extracellular Ca(2+) entry. Thus, α1A-AR activation induced the trafficking of AQP5 to the APM and LPM via the Ca(2+)/ cyclic guanosine monophosphate (cGMP)/PKG signaling pathway, which is associated with store-operated Ca(2+) entry.
Keywords: aquaporin-5; calcium; hypotonicity; protein G kinase; α1A-adrenoceptor; α1B-adrenoceptor; α1D-adrenoceptor.
Figures


References
-
- Raina S., Preston G.M., Guggino W.B., Agre P. Molecular cloning and characterization of an aquaporin cDNA from salivary, lacrimal, and respiratory tissues. J. Biol. Chem. 1995;270:1908–1912. - PubMed
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases
Research Materials
Miscellaneous

