Use of Different Proteases to Obtain Flaxseed Protein Hydrolysates with Antioxidant Activity
- PMID: 27367678
- PMCID: PMC4964403
- DOI: 10.3390/ijms17071027
Use of Different Proteases to Obtain Flaxseed Protein Hydrolysates with Antioxidant Activity
Abstract
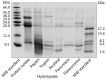
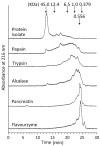
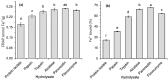
The antioxidant activity of flaxseed protein hydrolysates obtained using five different enzymes was evaluated. Proteins were isolated from flaxseed cake and were separately treated with papain, trypsin, pancreatin, Alcalase and Flavourzyme. The degree of hydrolysis (DH) was determined as the percentage of cleaved peptide bonds using a spectrophotometric method with o-phthaldialdehyde. The distribution of the molecular weights (MW) of the hydrolysis products was profiled using Tricine-sodium dodecyl sulfate-polyacrylamide gel electrophoresis (Tricine-SDS-PAGE) and size exclusion-high performance liquid chromatography (SE-HPLC) separations. The antioxidant activities of the protein isolate and hydrolysates were probed for their radical scavenging activity using 2,2'-azino-bis-(3-ethylbenzothiazoline-6-sulfonate) radical cation (ABTS(•+)) and photochemiluminescence (PCL-ACL) assays, and for their ferric reducing antioxidant power (FRAP) and ability to bind Fe(2+). The hydrolysates were more effective as antioxidants than the protein isolate in all systems. The PCL-ACL values of the hydrolysates ranged from 7.2 to 35.7 μmol Trolox/g. Both the FRAP and ABTS(•+) scavenging activity differed among the hydrolysates to a lower extent, with the ranges of 0.20-0.24 mmol Fe(2+)/g and 0.17-0.22 mmol Trolox/g, respectively. The highest chelating activity (71.5%) was noted for the pancreatin hydrolysate. In general, the hydrolysates obtained using Alcalase and pancreatin had the highest antioxidant activity, even though their DH (15.4% and 29.3%, respectively) and the MW profiles of the peptides varied substantially. The O₂(•-) scavenging activity and the ability to chelate Fe(2+) of the Flavourzyme hydrolysate were lower than those of the Alcalase and pancreatin hydrolysates. Papain was the least effective in releasing the peptides with antioxidant activity. The study showed that the type of enzyme used for flaxseed protein hydrolysis determines the antioxidant activity of the hydrolysates.
Keywords: antioxidant activity; enzymatic hydrolysis; flaxseed cake; proteases; protein hydrolysates.
Figures


Similar articles
-
Influence of spray drying encapsulation on the retention of antioxidant properties and microstructure of flaxseed protein hydrolysates.Colloids Surf B Biointerfaces. 2019 Jun 1;178:421-429. doi: 10.1016/j.colsurfb.2019.03.038. Epub 2019 Mar 19. Colloids Surf B Biointerfaces. 2019. PMID: 30908998
-
Antioxidant activities of bambara groundnut (Vigna subterranea) protein hydrolysates and their membrane ultrafiltration fractions.Food Funct. 2016 May 18;7(5):2431-7. doi: 10.1039/c6fo00057f. Epub 2016 May 9. Food Funct. 2016. PMID: 27156453
-
Generation, Fractionation, and Characterization of Iron-Chelating Protein Hydrolysate from Palm Kernel Cake Proteins.J Food Sci. 2016 Feb;81(2):C341-7. doi: 10.1111/1750-3841.13200. Epub 2015 Dec 31. J Food Sci. 2016. PMID: 26720491
-
Ultrasound microwave-assisted enzymatic production and characterisation of antioxidant peptides from sweet potato protein.Ultrason Sonochem. 2020 Dec;69:105262. doi: 10.1016/j.ultsonch.2020.105262. Epub 2020 Jul 17. Ultrason Sonochem. 2020. PMID: 32707458 Review.
-
Unconventional microbial proteases as promising tools for the production of bioactive protein hydrolysates.Crit Rev Food Sci Nutr. 2024;64(14):4714-4745. doi: 10.1080/10408398.2022.2145262. Epub 2022 Nov 15. Crit Rev Food Sci Nutr. 2024. PMID: 36377687 Review.
Cited by
-
Optimization of enzymatic hydrolysis of red tilapia scales (Oreochromis sp.) to obtain bioactive peptides.Biotechnol Rep (Amst). 2021 Apr 3;30:e00611. doi: 10.1016/j.btre.2021.e00611. eCollection 2021 Jun. Biotechnol Rep (Amst). 2021. PMID: 33912403 Free PMC article.
-
Effect of Protease Type and Peptide Size on the In Vitro Antioxidant, Antihypertensive and Anti-Diabetic Activities of Eggplant Leaf Protein Hydrolysates.Foods. 2021 May 18;10(5):1112. doi: 10.3390/foods10051112. Foods. 2021. PMID: 34069802 Free PMC article.
-
Effect of Alcalase Modification of Yellow Lupin (Lupinus luteus L.) Protein Isolate on Some Functional Properties and Antioxidant Activity.Int J Food Sci. 2022 Nov 18;2022:6187441. doi: 10.1155/2022/6187441. eCollection 2022. Int J Food Sci. 2022. PMID: 36438167 Free PMC article.
-
Recycled Sericin Hydrolysates Modified by Alcalase® Suppress Melanogenesis in Human Melanin-Producing Cells via Modulating MITF.Int J Mol Sci. 2022 Apr 1;23(7):3925. doi: 10.3390/ijms23073925. Int J Mol Sci. 2022. PMID: 35409284 Free PMC article.
-
Bioprocessing of Functional Ingredients from Flaxseed.Molecules. 2018 Sep 24;23(10):2444. doi: 10.3390/molecules23102444. Molecules. 2018. PMID: 30250012 Free PMC article. Review.
References
-
- Pihlanto A. Antioxidative peptides derived from milk proteins. Int. Dairy J. 2006;16:1306–1314. doi: 10.1016/j.idairyj.2006.06.005. - DOI
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Miscellaneous

