Supplementation of Reduced Gluten Barley Diet with Oral Prolyl Endopeptidase Effectively Abrogates Enteropathy-Associated Changes in Gluten-Sensitive Macaques
- PMID: 27367722
- PMCID: PMC4963877
- DOI: 10.3390/nu8070401
Supplementation of Reduced Gluten Barley Diet with Oral Prolyl Endopeptidase Effectively Abrogates Enteropathy-Associated Changes in Gluten-Sensitive Macaques
Abstract
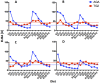
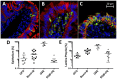
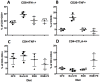
Celiac disease (CD) is an autoimmune disorder that affects approximately three million people in the United States. Furthermore, non-celiac gluten sensitivity (NCGS) affects an estimated additional 6% of the population, e.g., 20 million in the U.S. The only effective treatment of CD and NCGS requires complete removal of gluten sources from the diet. While required adherence to a gluten-free diet (GFD) is extremely difficult to accomplish, efforts to develop additional supportive treatments are needed. To facilitate these efforts, we developed a gluten-sensitive (GS) rhesus macaque model to study the effects of novel therapies. Recently reported results from phase one of this project suggest that partial improvement-but not remission-of gluten-induced disease can be accomplished by 100-fold reduction of dietary gluten, i.e., 200 ppm-by replacement of conventional dietary sources of gluten with a mutant, reduced gluten (RG) barley (lys3a)-derived source. The main focus of this (phase two) study was to determine if the inflammatory effects of the residual gluten in lys3a mutant barley grain could be further reduced by oral supplementation with a prolylendopeptidase (PE). Results reveal that PE supplementation of RG barley diet induces more complete immunological, histopathological and clinical remission than RG barley diet alone. The combined effects of RG barley diet and PE supplementation resulted in a further decrease of inflammatory mediators IFN-γ and TNF secretion by peripheral lymphocytes, as well as decreased plasma anti-gliadin and anti-intestinal tissue transglutaminase (TG2) antibodies, diminished active caspase production in small intestinal mucosa, and eliminated clinical diarrhea-all comparable with a gluten-free diet induced remission. In summary, the beneficial results of a combined RG barley and PE administration in GS macaques may warrant the investigation of similar synergistic approaches.
Keywords: IL-15; celiac; gluten; gluten-free; gluten-sensitive enteropathy; glutenase; oral supplement; protease; rhesus macaque.
Figures



References
-
- Tack G.J., van de Water J.M., Bruins M.J., Kooy-Winkelaar E.M., van Bergen J., Bonnet P., Vreugdenhil A.C., Korponay-Szabo I., Edens L., von Blomberg B.M., et al. Consumption of gluten with gluten-degrading enzyme by celiac patients: A pilot study. World J. Gastroenterol. 2013;19:5837–5847. doi: 10.3748/wjg.v19.i35.5837. - DOI - PMC - PubMed
-
- Wen S., Wen N., Pang J., Langen G., Brew-Appiah R.A., Mejias J.H., Osorio C., Yang M., Gemini R., Moehs C.P., et al. Structural genes of wheat and barley 5-methylcytosine DNA glycosylases and their potential applications for human health. Proc. Natl. Acad. Sci. USA. 2012;109:20543–20548. doi: 10.1073/pnas.1217927109. - DOI - PMC - PubMed
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical

