A Review of Glass-Ionomer Cements for Clinical Dentistry
- PMID: 27367737
- PMCID: PMC5040989
- DOI: 10.3390/jfb7030016
A Review of Glass-Ionomer Cements for Clinical Dentistry
Abstract
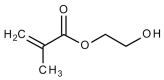
This article is an updated review of the published literature on glass-ionomer cements and covers their structure, properties and clinical uses within dentistry, with an emphasis on findings from the last five years or so. Glass-ionomers are shown to set by an acid-base reaction within 2-3 min and to form hard, reasonably strong materials with acceptable appearance. They release fluoride and are bioactive, so that they gradually develop a strong, durable interfacial ion-exchange layer at the interface with the tooth, which is responsible for their adhesion. Modified forms of glass-ionomers, namely resin-modified glass-ionomers and glass carbomer, are also described and their properties and applications covered. Physical properties of the resin-modified glass-ionomers are shown to be good, and comparable with those of conventional glass-ionomers, but biocompatibility is somewhat compromised by the presence of the resin component, 2 hydroxyethyl methacrylate. Properties of glass carbomer appear to be slightly inferior to those of the best modern conventional glass-ionomers, and there is not yet sufficient information to determine how their bioactivity compares, although they have been formulated to enhance this particular feature.
Keywords: bioactivity; clinical applications; fluoride release; glass carbomer; glass-ionomer cement; resin-modified.
Conflict of interest statement
The authors declare no conflict of interest.
Figures
References
-
- Mount G.J. Color Atlas of Glass Ionomer Cement. 2nd ed. Martin Dunitz; London, UK: 2002.
-
- Wilson A.D., Kent B.E. The glass-ionomer cement, a new translucent cement for dentistry. J. Appl. Chem. Biotechnol. 1971;21:313. doi: 10.1002/jctb.5020211101. - DOI
-
- ISO 9917–1: Dental Water Based Cements. International Organization for Standardization; Geneva, Switzerland: 2003.
-
- McLean J.W., Nicholson J.W., Wilson A.D. Guest Editorial: Proposed nomenclature for glass-ionomer dental cements and related materials. Quintessence Int. 1994;25:587–589. - PubMed
-
- Ellis J., Wilson A.D. Polyphosphonate cements: A new class of dental materials. J. Mater. Sci. Lett. 1990;9:1058–1060. doi: 10.1007/BF00727876. - DOI
Publication types
LinkOut - more resources
Full Text Sources
Other Literature Sources