Standardized Rehabilitation and Hospital Length of Stay Among Patients With Acute Respiratory Failure: A Randomized Clinical Trial
- PMID: 27367766
- PMCID: PMC6657499
- DOI: 10.1001/jama.2016.7201
Standardized Rehabilitation and Hospital Length of Stay Among Patients With Acute Respiratory Failure: A Randomized Clinical Trial
Abstract
Importance: Physical rehabilitation in the intensive care unit (ICU) may improve the outcomes of patients with acute respiratory failure.
Objective: To compare standardized rehabilitation therapy (SRT) to usual ICU care in acute respiratory failure.
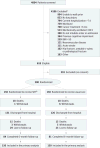
Design, setting, and participants: Single-center, randomized clinical trial at Wake Forest Baptist Medical Center, North Carolina. Adult patients (mean age, 58 years; women, 55%) admitted to the ICU with acute respiratory failure requiring mechanical ventilation were randomized to SRT (n=150) or usual care (n=150) from October 2009 through May 2014 with 6-month follow-up.
Interventions: Patients in the SRT group received daily therapy until hospital discharge, consisting of passive range of motion, physical therapy, and progressive resistance exercise. The usual care group received weekday physical therapy when ordered by the clinical team. For the SRT group, the median (interquartile range [IQR]) days of delivery of therapy were 8.0 (5.0-14.0) for passive range of motion, 5.0 (3.0-8.0) for physical therapy, and 3.0 (1.0-5.0) for progressive resistance exercise. The median days of delivery of physical therapy for the usual care group was 1.0 (IQR, 0.0-8.0).
Main outcomes and measures: Both groups underwent assessor-blinded testing at ICU and hospital discharge and at 2, 4, and 6 months. The primary outcome was hospital length of stay (LOS). Secondary outcomes were ventilator days, ICU days, Short Physical Performance Battery (SPPB) score, 36-item Short-Form Health Surveys (SF-36) for physical and mental health and physical function scale score, Functional Performance Inventory (FPI) score, Mini-Mental State Examination (MMSE) score, and handgrip and handheld dynamometer strength.
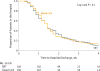
Results: Among 300 randomized patients, the median hospital LOS was 10 days (IQR, 6 to 17) for the SRT group and 10 days (IQR, 7 to 16) for the usual care group (median difference, 0 [95% CI, -1.5 to 3], P = .41). There was no difference in duration of ventilation or ICU care. There was no effect at 6 months for handgrip (difference, 2.0 kg [95% CI, -1.3 to 5.4], P = .23) and handheld dynamometer strength (difference, 0.4 lb [95% CI, -2.9 to 3.7], P = .82), SF-36 physical health score (difference, 3.4 [95% CI, -0.02 to 7.0], P = .05), SF-36 mental health score (difference, 2.4 [95% CI, -1.2 to 6.0], P = .19), or MMSE score (difference, 0.6 [95% CI, -0.2 to 1.4], P = .17). There were higher scores at 6 months in the SRT group for the SPPB score (difference, 1.1 [95% CI, 0.04 to 2.1, P = .04), SF-36 physical function scale score (difference, 12.2 [95% CI, 3.8 to 20.7], P = .001), and the FPI score (difference, 0.2 [95% CI, 0.04 to 0.4], P = .02).
Conclusions and relevance: Among patients hospitalized with acute respiratory failure, SRT compared with usual care did not decrease hospital LOS.
Trial registration: clinicaltrials.gov Identifier: NCT00976833.
Conflict of interest statement
Figures
Comment in
-
The Challenging Task of Improving the Recovery of ICU Survivors.JAMA. 2016 Jun 28;315(24):2671-2. doi: 10.1001/jama.2016.7211. JAMA. 2016. PMID: 27367765 No abstract available.
-
Daily rehabilitation improves physical function at 6 months, but not hospital length of stay, in patients with acute respiratory failure [synopsis].J Physiother. 2017 Jan;63(1):49. doi: 10.1016/j.jphys.2016.10.005. Epub 2016 Nov 9. J Physiother. 2017. PMID: 27964956 No abstract available.
-
Daily rehabilitation improves physical function at 6 months, but not hospital length of stay, in patients with acute respiratory failure [commentary].J Physiother. 2017 Jan;63(1):49. doi: 10.1016/j.jphys.2016.10.004. Epub 2016 Nov 9. J Physiother. 2017. PMID: 27964961 No abstract available.
-
Recovery from Critical Illness: Physical Rehabilitation in the Intensive Care Unit, Timing of Persistent Critical Illness, and Caregiver Outcomes.Am J Respir Crit Care Med. 2017 Oct 15;196(8):1068-1070. doi: 10.1164/rccm.201704-0828RR. Am J Respir Crit Care Med. 2017. PMID: 28841330 No abstract available.
References
-
- Hermans G, Van Mechelen H, Clerckx B, et al. Acute outcomes and 1-year mortality of intensive care unit-acquired weakness: a cohort study and propensity-matched analysis. Am J Respir Crit Care Med. 2014;190(4):410–420. - PubMed
-
- Herridge MS, Tansey CM, Matté A, et al. Canadian Critical Care Trials Group Functional disability 5 years after acute respiratory distress syndrome. N Engl J Med. 2011;364(14):1293–1304. - PubMed
-
- Bailey P, Thomsen GE, Spuhler VJ, et al. Early activity is feasible and safe in respiratory failure patients. Crit Care Med. 2007;35(1):139–145. - PubMed
Publication types
MeSH terms
Associated data
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical

