Recent advances in understanding renal ammonia metabolism and transport
- PMID: 27367914
- PMCID: PMC4974126
- DOI: 10.1097/MNH.0000000000000255
Recent advances in understanding renal ammonia metabolism and transport
Abstract
Purpose of review: The purpose of this review is to provide a succinct description of the recent findings that advance our understanding of the fundamental renal process of ammonia metabolism and transport in conditions relevant to the clinician.
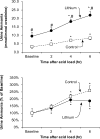
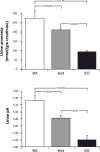
Recent findings: Recent studies advance our understanding of renal ammonia metabolism. Mechanisms through which chronic kidney disease and altered dietary protein intake alter ammonia excretion have been identified. Lithium, although it can acutely cause distal renal tubular acidosis, was shown with long-term use to increase urinary ammonia excretion, and this appeared to be mediated, at least in part, by increased Rhcg expression. Gene deletion studies showed that the ammonia recycling enzyme, glutamine synthetase, has a critical role in normal-stimulated and acidosis-stimulated ammonia metabolism and that the proximal tubule basolateral bicarbonate transporter, NBCe1, is necessary for normal ammonia metabolism. Finally, our understanding of the molecular ammonia species, NH3 versus NH4, transported by Rh glycoproteins continues to be advanced.
Summary: Fundamental studies have been recently published that advance our understanding of the regulation of ammonia metabolism in clinically important circumstances, and our understanding of the mechanisms and regulation of proximal tubule ammonia generation, and the mechanisms through which Rh glycoproteins contribute to ammonia secretion.
Conflict of interest statement
The authors have no financial, consultant, institutional or other relationship that might lead to bias or a conflict of interest in regards to information presented in this review.
Figures

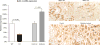

References
-
- Mitch WE. Metabolic and clinical consequences of metabolic acidosis. J Nephrol. 2006;19:S70–S75. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Research Materials

