Review of Mouse Models of Graves' Disease and Orbitopathy-Novel Treatment by Induction of Tolerance
- PMID: 27368808
- PMCID: PMC5346423
- DOI: 10.1007/s12016-016-8562-7
Review of Mouse Models of Graves' Disease and Orbitopathy-Novel Treatment by Induction of Tolerance
Abstract
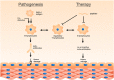
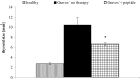
Various approaches have been used to model human Graves' disease in mice, including transfected fibroblasts, and plasmid or adenoviral immunisations with the extracellular A subunit of the human thyrotropin receptor (TSHR). Some of these models were only observed for a short time period or were self-limiting. A long-term model for human Graves' disease was established in mice using continuing immunisations (4-weekly injections) with recombinant adenovirus expressing TSHR. Generation of TSHR binding cAMP-stimulatory antibodies, thyroid enlargement and alterations, elevated serum thyroxin levels, tachycardia and cardiac hypertrophy were maintained for at least 9 months in all Ad-TSHR-immunised mice. Here, we show that these mice suffer from orbitopathy, which was detected by serial orbital sectioning and histomorphometry. Attempts to treat established Graves' disease in preclinical mouse model studies have included small molecule allosteric antagonists and specific antagonist antibodies which were isolated from hypothyroid patients. In addition, novel peptides have been conceived which mimic the cylindrical loops of the TSHR leucine-rich repeat domain, in order to re-establish tolerance toward the antigen. Here, we show preliminary results that one set of these peptides improves or even cures all signs and symptoms of Graves' disease in mice after six consecutive monthly injections. First beneficial effects were observed 3-4 months after starting these therapies. In immunologically naïve mice, administration of the peptides did not induce any immune response.
Keywords: Autoimmunity; Graves’ disease; Peptides; Thyreotropin receptor; Tolerance.
Conflict of interest statement
All applicable international, national and institutional guidelines for the care and use of animals were followed. All animal experiments were approved by the local animal welfare authority and Ethics committee at the Regierung von Oberbayern (Government of Upper Bavaria) in Munich, Germany (no. 55.2-1-54-2531-25-12) and carried out in accordance to the European Commission guidelines. All procedures performed in studies involving animals were in accordance with the ethical standards of the institution or practice at which the studies were conducted.
Figures





References
-
- Abraham-Nordling M, Törring O, Hamberger B, Lundell G, Tallstedt L, Calissendorf J, Wallin G. Graves’ disease: a long-term quality of life follow up of patients randomized to treatment with antithyroid drugs, radioiodine or surgery. Thyroid. 2005;15:1279–1285. doi: 10.1089/thy.2005.15.1279. - DOI - PubMed
-
- Shimojo N, Kohno Y, Yamaguchi KI, Kikuoka SI, Hoshioka A, Niimi H, Hirai A, Tamura Y, Saito Y, Kohn LD, Tahara K. Induction of Graves-like disease in mice by immunization with fibroblasts transfected with the thyreotropin receptor and a class II molecule. Proc Natl Acad Sci. 1996;93:11074–11079. doi: 10.1073/pnas.93.20.11074. - DOI - PMC - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources

