Palbociclib in Combination With Fulvestrant in Women With Hormone Receptor-Positive/HER2-Negative Advanced Metastatic Breast Cancer: Detailed Safety Analysis From a Multicenter, Randomized, Placebo-Controlled, Phase III Study (PALOMA-3)
- PMID: 27368881
- PMCID: PMC5061543
- DOI: 10.1634/theoncologist.2016-0097
Palbociclib in Combination With Fulvestrant in Women With Hormone Receptor-Positive/HER2-Negative Advanced Metastatic Breast Cancer: Detailed Safety Analysis From a Multicenter, Randomized, Placebo-Controlled, Phase III Study (PALOMA-3)
Abstract
Background: Palbociclib enhances endocrine therapy and improves clinical outcomes in hormone receptor (HR)-positive/human epidermal growth factor receptor 2 (HER2)-negative metastatic breast cancer (MBC). Because this is a new target, it is clinically important to understand palbociclib's safety profile to effectively manage toxicity and optimize clinical benefit.
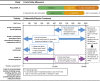
Materials and methods: Patients with endocrine-resistant, HR-positive/HER2-negative MBC (n = 521) were randomly assigned 2:1 to receive fulvestrant (500 mg intramuscular injection) with or without goserelin with oral palbociclib (125 mg daily; 3 weeks on/1 week off) or placebo. Safety assessments at baseline and day 1 of each cycle included blood counts on day 15 for the first 2 cycles. Hematologic toxicity was assessed by using laboratory data.
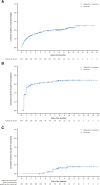
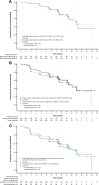
Results: A total of 517 patients were treated (palbociclib, n = 345; placebo, n = 172); median follow-up was 8.9 months. With palbociclib, neutropenia was the most common grade 3 (55%) and 4 (10%) adverse event; median times to onset and duration of grade ≥3 episodes were 16 and 7 days, respectively. Asian ethnicity and below-median neutrophil counts at baseline were significantly associated with an increased chance of developing grade 3-4 neutropenia with palbociclib. Dose modifications for grade 3-4 neutropenia had no adverse effect on progression-free survival. In the palbociclib arm, febrile neutropenia occurred in 3 (<1%) patients. The percentage of grade 1-2 infections was higher than in the placebo arm. Grade 1 stomatitis occurred in 8% of patients.
Conclusion: Palbociclib plus fulvestrant treatment was well-tolerated, and the primary toxicity of asymptomatic neutropenia was effectively managed by dose modification without apparent loss of efficacy. This study appears at ClinicalTrials.gov, NCT01942135.
Implications for practice: Treatment with palbociclib in combination with fulvestrant was generally safe and well-tolerated in patients with hormone receptor (HR)-positive metastatic breast cancer. Consistent with the drug's proposed mechanism of action, palbociclib-related neutropenia differs in its clinical time course, patterns, and consequences from those seen with chemotherapy. Neutropenia can be effectively managed by a dose reduction, interruption, or cycle delay without compromising efficacy. A significant efficacy gain and a favorable safety profile support the consideration of incorporating palbociclib into the routine management of HR-positive/human epidermal growth factor receptor 2-negative metastatic breast cancer.
Keywords: Cyclin-dependent kinase 4; Cyclin-dependent kinase 6; Neutropenia; Palbociclib.
©AlphaMed Press.
Conflict of interest statement
of potential conflicts of interest may be found at the end of this article.
Figures

Comment in
-
Safety Analysis of Premenopausal Breast Cancer Patients in PALOMA-3 Study: Is It Worth Mentioning?Oncologist. 2017 Jun;22(6):754. doi: 10.1634/theoncologist.2016-0446. Oncologist. 2017. PMID: 28611237 Free PMC article.
-
In Reply.Oncologist. 2017 Jun;22(6):754-755. doi: 10.1634/theoncologist.2016-0469. Oncologist. 2017. PMID: 28611238 Free PMC article.
References
-
- Finn RS, Crown JP, Lang I, et al. The cyclin-dependent kinase 4/6 inhibitor palbociclib in combination with letrozole versus letrozole alone as first-line treatment of oestrogen receptor-positive, HER2-negative, advanced breast cancer (PALOMA-1/TRIO-18): A randomised phase 2 study. Lancet Oncol. 2015;16:25–35. - PubMed
-
- Cristofanilli M, Turner NC, Bondarenko I, et al. Fulvestrant plus palbociclib versus fulvestrant plus placebo for treatment of hormone-receptor-positive, HER2-negative metastatic breast cancer that progressed on previous endocrine therapy (PALOMA-3): Final analysis of the multicentre, double-blind, phase 3 randomised controlled trial. Lancet Oncol. 2016;17:425–439. - PubMed
-
- Turner NC, Ro J, André F, et al. Palbociclib in hormone-receptor-positive advanced breast cancer. N Engl J Med. 2015;373:209–219. - PubMed
Publication types
MeSH terms
Substances
Associated data
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Research Materials
Miscellaneous

