Structure/function correlations over binuclear non-heme iron active sites
- PMID: 27369780
- PMCID: PMC5010389
- DOI: 10.1007/s00775-016-1372-9
Structure/function correlations over binuclear non-heme iron active sites
Abstract
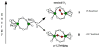
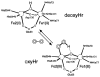
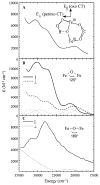
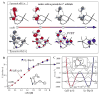
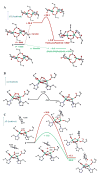
Binuclear non-heme iron enzymes activate O2 to perform diverse chemistries. Three different structural mechanisms of O2 binding to a coupled binuclear iron site have been identified utilizing variable-temperature, variable-field magnetic circular dichroism spectroscopy (VTVH MCD). For the μ-OH-bridged Fe(II)2 site in hemerythrin, O2 binds terminally to a five-coordinate Fe(II) center as hydroperoxide with the proton deriving from the μ-OH bridge and the second electron transferring through the resulting μ-oxo superexchange pathway from the second coordinatively saturated Fe(II) center in a proton-coupled electron transfer process. For carboxylate-only-bridged Fe(II)2 sites, O2 binding as a bridged peroxide requires both Fe(II) centers to be coordinatively unsaturated and has good frontier orbital overlap with the two orthogonal O2 π* orbitals to form peroxo-bridged Fe(III)2 intermediates. Alternatively, carboxylate-only-bridged Fe(II)2 sites with only a single open coordination position on an Fe(II) enable the one-electron formation of Fe(III)-O2 (-) or Fe(III)-NO(-) species. Finally, for the peroxo-bridged Fe(III)2 intermediates, further activation is necessary for their reactivities in one-electron reduction and electrophilic aromatic substitution, and a strategy consistent with existing spectral data is discussed.
Keywords: Binuclear non-heme iron enzymes; Frontier molecular orbitals; O2 activation; Peroxide activation; Variable-temperature, variable-field magnetic circular dichroism.
Figures



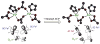


References
-
- Solomon EI, Brunold TC, Davis MI, Kemsley JN, Lee SK, Lehnert N, Neese F, Skulan AJ, Yang YS, Zhou J. Chem Rev. 2000;100:235–350. - PubMed
-
- Ziehl M, He J, Dahse H-M, Hertweck C. Angew Chem Int Ed Engl. 2005;44:1202–1205. - PubMed
-
- Yang B, Hodgkinson A, Millward BA, Demaine AG. Int J Diabetes Mellit. 2010;2:169–174.
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Research Materials
Miscellaneous

