Long-term analysis of the NOA-04 randomized phase III trial of sequential radiochemotherapy of anaplastic glioma with PCV or temozolomide
- PMID: 27370396
- PMCID: PMC5063521
- DOI: 10.1093/neuonc/now133
Long-term analysis of the NOA-04 randomized phase III trial of sequential radiochemotherapy of anaplastic glioma with PCV or temozolomide
Erratum in
-
Corrigendum.Neuro Oncol. 2016 Nov;18(11):e1. doi: 10.1093/neuonc/now228. Neuro Oncol. 2016. PMID: 27738185 Free PMC article. No abstract available.
Abstract
Background: Optimal treatment and precise classification for anaplastic glioma are needed.
Methods: The objective for long-term follow-up of NOA-04 is to optimize the treatment sequence for patients with anaplastic gliomas. Patients were randomized 2:1:1 to receive the standard radiotherapy (RT) (arm A), procarbazine, lomustine and vincristine (PCV) (arm B1), or temozolomide (TMZ) (arm B2).
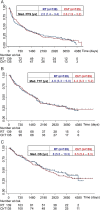
Results: Primary endpoint was time-to-treatment-failure (TTF), defined as progression after 2 lines of therapy or any time before if no further therapy was administered. Exploratory analyses examined associations of molecular marker status with TTF, progression-free survival (PFS), and overall survival (OS). At 9.5 (95% CI: 8.6-10.2) years, no difference between arms (A vs B1/B2) was observed: median TTF (4.6 [3.4-5.1] y vs 4.4 [3.3-5.3) y), PFS (2.5 [1.3-3.5] y vs 2.7 [1.9-3.2] y), and OS (8 [5.5-10.3] y vs 6.5 [5.4-8.3] y). Oligodendroglial versus astrocytic histology-but more so the subgroups according to CpG island methylator phenotype (CIMP) and 1p/19q co-deletion status-revealed a strong prognostic value of CIMPpos with (CIMPcodel) versus without 1p/19 co-deletion (CIMPnon-codel) versus CIMPneg. but no differential efficacy of RT versus chemotherapy for any of the endpoints. PFS was better for PCV- than for TMZ-treated patients with CIMPcodel tumors (HR B1 vs B2 0.39 [0.17-0.92], P = .031). In CIMPneg. tumors, hypermethylation of the O6-methyl-guanyl-DNA methyltransferase promoter (MGMT) provided a risk reduction for PFS with chemotherapy.
Conclusions: There is no differential activity of primary chemotherapy versus RT in any subgroup of anaplastic glioma. Molecular diagnosis is superior to histology.
Trial registration: clinicaltrials.gov Identifier: NCT00717210.
Keywords: 1p/19q; CIMP; MGMT; anaplastic gliomas.
© The Author(s) 2016. Published by Oxford University Press on behalf of the Society for Neuro-Oncology. All rights reserved. For permissions, please e-mail: journals.permissions@oup.com.
Figures


Comment in
-
Biomarkers in NOA-04: another piece to the puzzle.Neuro Oncol. 2016 Nov;18(11):1467-1469. doi: 10.1093/neuonc/now210. Neuro Oncol. 2016. PMID: 27738177 Free PMC article. No abstract available.
-
Treatment of WHO Grade 2 and 3 Gliomas With Potentially Favorable Survival: Is Monotherapy Obsolete?Int J Radiat Oncol Biol Phys. 2019 Mar 1;103(3):533-536. doi: 10.1016/j.ijrobp.2018.08.025. Epub 2019 Feb 1. Int J Radiat Oncol Biol Phys. 2019. PMID: 31088778 No abstract available.
References
-
- van den Bent MJ, Brandes AA, Taphoorn MJ et al. . Adjuvant procarbazine, lomustine, and vincristine chemotherapy in newly diagnosed anaplastic oligodendroglioma: long-term follow-up of EORTC brain tumor group study 26951. J Clin Oncol. 2013;31(3):344–350. - PubMed
-
- Buckner JC, Pugh SL, Shaw EG et al. . Phase III study of radiation therapy (RT) with or without procarbazine, CCNU, and vincristine (PCV) in low-grade glioma: RTOG 9802 with Alliance, ECOG, and SWOG. J Clin Oncol. 2014;32:5s (suppl; abstr 2000).
-
- Hartmann C, Hentschel B, Wick W et al. . Patients with IDH1 wild type anaplastic astrocytomas exhibit worse prognosis than IDH1-mutated glioblastomas, and IDH1 mutation status accounts for the unfavorable prognostic effect of higher age: implications for classification of gliomas. Acta Neuropathol. 2010;120(6):707–718. - PubMed
-
- Wiestler B, Capper D, Sill M et al. . Integrated DNA methylation and copy-number profiling identify three clinically and biologically relevant groups of anaplastic glioma. Acta Neuropathol. 2014;128(4):561–571. - PubMed
Publication types
MeSH terms
Substances
Supplementary concepts
Associated data
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Research Materials

