Fluphenazine (oral) versus atypical antipsychotics for schizophrenia
- PMID: 27370402
- PMCID: PMC6474115
- DOI: 10.1002/14651858.CD010832.pub2
Fluphenazine (oral) versus atypical antipsychotics for schizophrenia
Abstract
Background: Fluphenazine is a typical antipsychotic drug from the phenothiazine group of antipsychotics. It has been commonly used in the treatment of schizophrenia, however, with the advent of atypical antipsychotic medications, use has declined over the years.
Objectives: To measure the outcomes (both beneficial and harmful) of the clinical effectiveness, safety and cost-effectiveness of oral fluphenazine versus atypical antipsychotics for schizophrenia.
Search methods: We searched the Cochrane Central Register of Studies (25 April 2013). For the economic search, we searched the Cochrane Schizophrenia Group Health Economic Database (CSzGHED) on 31 January 2014 SELECTION CRITERIA: All randomised controlled trials (RCTs) comparing fluphenazine (oral) with any other oral atypical antipsychotics.
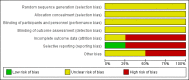
Data collection and analysis: Review authors worked independently to inspect citations and assess the quality of the studies and to extract data. For homogeneous dichotomous data we calculated the risk ratio (RR) and 95% confidence interval (CI), and calculated the mean differences (MDs) for continuous data. We assessed risk of bias for included studies and used GRADE (Grading of Recommendations Assessment, Development and Evaluation) to rate the quality of the evidence.
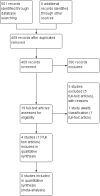
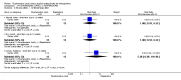
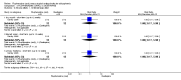
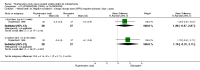
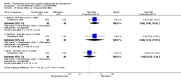
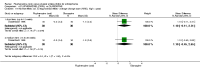
Main results: Four studies randomising a total of 202 people with schizophrenia are included. Oral fluphenazine was compared with oral amisulpride, risperidone, quetiapine and olanzapine.Comparing oral fluphenazine with amisulpride, there was no difference between groups for mental state using the Brief Psychiatric Rating Scale (BPRS) (1 RCT, n = 57, MD 5.10 95% CI -2.35 to 12.55, very low-quality evidence), nor was there any difference in numbers leaving the study early for any reason (2 RCTs, n = 98, RR 1.19 95% CI 0.63 to 2.28, very low-quality evidence). More people required concomitant anticholinergic medication in the fluphenazine group compared to amisulpride (1 RCT, n = 36, RR 7.82 95% CI 1.07 to 57.26, very low-quality evidence). No data were reported for important outcomes including relapse, changes in life skills, quality of life or cost-effectiveness.Comparing oral fluphenazine with risperidone, data showed no difference between groups for 'clinically important response' (1 RCT, n = 26, RR 0.67 95% CI 0.13 to 3.35, very low-quality evidence) nor leaving the study early due to inefficacy (1 RCT, n = 25, RR 1.08 95% CI 0.08 to 15.46, very low-quality evidence). No data were reported data for relapse; change in life skills; quality of life; extrapyramidal adverse effects; or cost-effectiveness.Once again there was no difference when oral fluphenazine was compared with quetiapine for clinically important response (1 RCT, n = 25, RR 0.62 95% CI 0.12 to 3.07, very low-quality evidence), nor leaving the study early for any reason (1 RCT, n = 25, RR 0.46 95% CI 0.05 to 4.46, very low-quality evidence). No data were reported for relapse; clinically important change in life skills; quality of life; extrapyramidal adverse effects; or cost-effectiveness.Compared to olanzapine, fluphenazine showed no superiority for clinically important response (1 RCT, n = 60, RR 1.33 95% CI 0.86 to 2.07, very low-quality evidence), in incidence of akathisia (1 RCT, n = 60, RR 3.00 95% CI 0.90 to 10.01, very low-quality evidence) or in people leaving the study early (1 RCT, n = 60, RR 3.00 95% CI 0.33 to 27.23, very low-quality evidence). No data were reported for relapse; change in life skills; quality of life; or cost-effectiveness.
Authors' conclusions: Measures of clinical response and mental state do not highlight differences between fluphenazine and amisulpride, risperidone, quetiapine or olanzapine. Largely measures of adverse effects are also unconvincing for substantive differences between fluphenazine and the newer drugs. All included trials carry a substantial risk of bias regarding reporting of adverse effects and this bias would have favoured the newer drugs. The four small short included studies do not provide much clear information about the relative merits or disadvantages of oral fluphenazine compared with newer atypical antipsychotics.
Conflict of interest statement
James Sampford: none known.
Stephanie Sampson: employed by the National Health Service to undertake Cochrane Programme Grant Reviews (SR ‐ 10/4001/15: Cost‐effective treatments and diagnostic approaches for people with schizophrenia within the NHS).
Sai Zhao: received payment from the NIHR funding detailed above.
Jun Xia: received payment from the NIHR funding detailed above.
Bao Guo Li: none known.
Vivek Furtado: none known
Figures










































Update of
- doi: 10.1002/14651858.CD010832
Similar articles
-
Chlorpromazine versus atypical antipsychotic drugs for schizophrenia.Cochrane Database Syst Rev. 2016 Apr 5;4(4):CD010631. doi: 10.1002/14651858.CD010631.pub2. Cochrane Database Syst Rev. 2016. PMID: 27045703 Free PMC article.
-
Risperidone (depot) for schizophrenia.Cochrane Database Syst Rev. 2016 Apr 14;4(4):CD004161. doi: 10.1002/14651858.CD004161.pub2. Cochrane Database Syst Rev. 2016. PMID: 27078222 Free PMC article.
-
Clozapine combined with different antipsychotic drugs for treatment-resistant schizophrenia.Cochrane Database Syst Rev. 2017 Mar 23;3(3):CD006324. doi: 10.1002/14651858.CD006324.pub3. Cochrane Database Syst Rev. 2017. PMID: 28333365 Free PMC article.
-
Aripiprazole versus other atypical antipsychotics for schizophrenia.Cochrane Database Syst Rev. 2014 Jan 2;2014(1):CD006569. doi: 10.1002/14651858.CD006569.pub5. Cochrane Database Syst Rev. 2014. PMID: 24385408 Free PMC article.
-
Cannabis and schizophrenia.Cochrane Database Syst Rev. 2014 Oct 14;2014(10):CD004837. doi: 10.1002/14651858.CD004837.pub3. Cochrane Database Syst Rev. 2014. PMID: 25314586 Free PMC article.
Cited by
-
Spectroscopic Properties and Biological Activity of Fluphenazine Conjugates with Gold Nanoparticles.Molecules. 2024 Dec 17;29(24):5948. doi: 10.3390/molecules29245948. Molecules. 2024. PMID: 39770038 Free PMC article.
-
Should we be Prescribing Fluphenazine Long-Acting Injectable Formulation?Curr Psychiatry Rep. 2025 Jun;27(6):408-414. doi: 10.1007/s11920-025-01610-y. Epub 2025 Apr 28. Curr Psychiatry Rep. 2025. PMID: 40289033 Free PMC article. Review.
-
Green Synthetic Strategies and Pharmaceutical Applications of Thiazine and its Derivatives: An Updated Review.Curr Pharm Biotechnol. 2024;25(9):1142-1166. doi: 10.2174/1389201025666230908141543. Curr Pharm Biotechnol. 2024. PMID: 37694776 Review.
-
Treatment resistant schizophrenia: a comprehensive survey of randomised controlled trials.BMC Psychiatry. 2014 Sep 12;14:253. doi: 10.1186/s12888-014-0253-4. BMC Psychiatry. 2014. PMID: 25227719 Free PMC article. Review.
-
Haloperidol versus first-generation antipsychotics for the treatment of schizophrenia and other psychotic disorders.Cochrane Database Syst Rev. 2015 Jan 16;1(1):CD009831. doi: 10.1002/14651858.CD009831.pub2. Cochrane Database Syst Rev. 2015. PMID: 25592299 Free PMC article.
References
References to studies included in this review
Boyer 1987 {published data only}
-
- Boyer P. Efficacy of low doses of atypical neuroleptics (benzamides) in defect states [Etude de l'efficacite de faibles doses de neuroleptiques atypiques (benzamides) dans les etats deficitaires]. Annales Medico‐Psychologiques 1986;144(6):593‐9. [MEDLINE: ] - PubMed
-
- Boyer P, Puech AJ. Determinants for clinical activity of neuroleptic drugs: chemical substances, doses, assessment tools. Psychiatrie and Psychobiologie 1987;2(4):296‐305.
-
- Pichot P, Boyer P. A double blind, controlled, multicenter trial of low dose amisulpride (solian (R) 50) versus low dose fluphenazine in the treatment of negative symptoms in chronic schizophrenia [Essai multicentrique controle, en double insu, amisulpride (solian (r) 50) contre fluphenazine a faibles doses dans le traitement du syndrome deficitaire des schizophrenies chroniques]. Annales de Psychiatrie 1988;3(3):312‐20.
-
- Pichot P, Boyer P. Controlled double‐blind multi‐centre trial of low dose amisulpride versus fluphenazine in the treatment of the negative syndrome of chronic schizophrenia. In: Borenstein P, et al. editor(s). Amisulpride. Paris: Expansion Scientifique Française, 1989:125–38.
Conley 2005 {published data only}
-
- Conley RR. New antipsychotic strategies: quetiapine and risperidone vs. fluphenazine in treatment resistant schizophrenia. Http://www.clinicaltrials.gov 2005.
-
- Conley RR, Kelly DL, Nelson MW, Richardson CM, Feldman S, Benham R, et al. Risperidone, quetiapine, and fluphenazine in the treatment of patients with therapy‐refractory schizophrenia. Schizophrenia Bulletin 2005;31(4):163‐8. - PubMed
-
- Kelly D Conley RR. Sexual side effects of quetiapine and risperidone compared with fluphenazine. Psychoneuroendocrinology 1999.
-
- Kelly DL, Conley RR. A randomised double‐blind 12‐week study of quetiapine, risperidone or fluphenazine on sexual functioning in people with schizophrenia. Psychoneuroendocrinology 2006;31(3):340‐6. - PubMed
-
- Kelly DL, Conley RR. Thyroid function in treatment‐resistant schizophrenia patients treated with quetiapine, risperidone, or fluphenazine. Journal of Clinical Psychiatry 2005;66(1):80‐4. - PubMed
Dossenbach 1998 {published data only}
-
- Dossenbach M, Friedel P, Jakovljevic M, Hotujac L, Folnegovic V, Uglesic B, et al. Olanzapine versus fluphenazine ‐ six weeks treatment of acute schizophrenia. 10th European Colleague of Neuropsychopharmacology Congress; 1997 September 13‐17; Vienna. 1997.
-
- Dossenbach M, Jakovljevic M, Folnegovi V, Uglesic B, Dodig G, Friedel P, et al. Olanzapine versus fluphenazine ‐ six weeks of treatment of anxiety symptoms during acute schizophrenia. Proceedings of the 10th European College of Neuropsychopharmacology Congress; 1997 Sep 13‐17; Vienna, Austria. 1998, issue 1‐2:203.
-
- Dossenbach MRK, Folnegovic‐Smalc V, Hotujac L, Uglesic B, Tollefson GD, Grundy SL, et al. Double‐blind, randomized comparison of olanzapine versus fluphenazine in the long‐term treatment of schizophrenia. Progress in Neuro‐Psychopharmacology and Biological Psychiatry 2004;28(2):311‐8. - PubMed
-
- Jakovljevic M, Dossenbach MR, Friedel P, Schausberger B, Grundy SL, Hotujac L, et al. Olanzapine versus fluphenazine in the acute (six week) treatment of schizophrenia. Psychiatria Danubina 1999; Vol. 11, issue 1‐2:3‐11.
-
- Jakovljevic M, Dossenbach MRK. Olanzapine versus fluphenazine in the acute (six week) treatment of schizophrenia. Psychiatria Danubina 1999; Vol. 11, issue 1‐2:3‐10.
Saletu 1994 {published data only}
-
- Saletu B, Kufferle B, Grunberger J, Foldes P, Topitz A, Anderer P. Clinical, EEG mapping and psychometric studies in negative schizophrenia: comparative trials with amisulpride and fluphenazine. Neuropsychobiology 1994;29(3):125‐35. - PubMed
References to studies excluded from this review
Boyer 1986 {published data only}
-
- Boyer P. Efficacy of low doses of atypical neuroleptics (benzamides) in defect states [Etude de l'efficacite de faibles doses de neuroleptiques atypiques (benzamides) dans les etats deficitaires]. Annales Medico‐Psychologiques 1986;144(6):593‐9. [MEDLINE: ] - PubMed
-
- Boyer P, Lecrubier Y, Puech AJ. Treatment of positive and negative symptoms: pharmacologic approaches. Modern Problems of PharmacoPsychiatry 1990;24:152‐74. [MEDLINE: ] - PubMed
-
- Pichot P, Boyer P. A controlled double‐blind multi‐centre trial of high dose amisulpride versus haloperidol in acute psychotic states. Amisulpride. Paris: Expansion Scientifique Francaise, 1989:83‐92.
-
- Pichot P, Boyer P. A double blind, controlled, multicenter trial of amisulpride versus high dose haloperidol in acute psychotic disorders. Annales de Psychiatrie 1988, issue 3:326‐32.
Boyer 1987a {published data only}
-
- Boyer P. Amisulpride in the treatment of negative schizophrenic symptoms: results of a double‐blind controlled trial versus placebo. Amisulpride. Paris: Expansion Scientifique Française, 1989:111‐23.
-
- Boyer P, Lecrubier Y, Puech AJ. Treatment of positive and negative symptoms: pharmacologic approaches. Modern Problems of PharmacoPsychiatry 1990;24:152‐74. [MEDLINE: ] - PubMed
-
- Boyer P, Puech A, Kossmann L, Lecrubier Y, Dewailly J. A controlled study versus placebo with low dose of amisulpride in the treatment of the purely deficit schizophrenia [Etude controlee versus placebo de faibles doses d'amisulpride dans le traitement des formes purement deficitaires de schizophrenie.]. L'Encephale 1989;15(2):300.
-
- Boyer P, Puech AJ, Lecrubier Y. Double blind trial versus placebo of low dose amisulpride (Solian 50) in schizophrenia with exclusively negative symptoms. Preliminary analysis of results [Etude en double insu contre placebo de l'amisulpride (Solian (r) 50) a faible dose chez des schizophrenes purement deficitaires. Premiere analyse des resultats]. Annales de Psychiatrie 1988;3(3):321‐5.
-
- Lecrubier Y, Puech AJ, Aubin F, Boyer P, Deyrieux B. Improvement by amisulpride of the negative syndrome in non‐psychotic subjects: a preliminary study. Psychiatrie and Psychobiologie 1988;3(5):329‐34. [MEDLINE: ]
Boyer 1996 {published data only}
-
- Boyer P, Turjanski S, Fleurot O. Amisulpride in the treatment of acute schizophrenia: a double‐blind comparison with haloperidol. Proceedings of the 20th Collegium Internationale Neuro‐Psychopharmacologicum Congress; 1996 Jun 23‐27; Melbourne, Australia. 1996.
-
- Möller HJ, Boyer P, Fleurot O, Rein W. Improvement of acute exacerbations of schizophrenia with amisulpride: a comparison with haloperidol. Psychopharmacology 1997;132(4):396‐401. [MEDLINE: ] - PubMed
-
- Möller HJ, Boyer P, Rein W, Eich FX. Treatment of schizophrenic patients with actue exacerbations: a double‐blind comparison of amisulpride and haloperidol. PharmacoPsychiatry 1997;30:199.
-
- Möller HJ, Boyer P, Turjanski S, Fleurot O. Amisulpride in the treatment of subchronic or chronic schizophrenia with acute exacerbation: a double‐blind comparison with haloperidol. Proceedings of the 8th Congress of the Association of European Psychiatrists; 1996 Jul 7‐12; London, UK. 1996.
Pickar 1992 {published data only}
-
- Pickar D, Owen RR, Litman RE, Konicki E, Gutierrez R, Rapaport MH. Clinical and biologic response to clozapine in patients with schizophrenia. Crossover comparison with fluphenazine. Archives of General Psychiatry 1992; Vol. 49, issue 5:345‐53. - PubMed
Ravanic 1996 {published data only}
-
- Ravanic DB, Djukic‐Dejanovic SM, Stojiljkovic M, Jankovic S, Paunovic VR, Bankovic D. Antipsychotic efficacy of clozapine vs fluphenazine in positive and negative schizophrenia syndrome. Journal of Neural Transmission 1996; Vol. 103:Xlvi.
References to studies awaiting assessment
Djukic‐Dejanovic 2002 {published data only}
-
- Djukic‐Dejanovic SM, Pantovic MM, Alexopulos C, Milovanovic DR, Paunovic VR, Ravanic DB. Clozapine vs. classical antipsychotics in schizophrenia. Proceedings of the 12th World Congress of Psychiatry; 2002 Aug 24‐29; Yokohama, Japan. 2002.
Additional references
Altman 1996
Andreasen 1982
-
- Andreasen NC. Negative symptoms in schizophrenia: definition and reliability. Archives of General Psychiatry 1982;39(7):784‐8. - PubMed
APA 2004
-
- Lehman AF, Lieberman JA, Dixon LB, McGlashan TH, Miller AL, Perkins DO, et al. Practice guideline for the treatment of patients with schizophrenia, second edition. American Journal of Psychiatry 2004;161(Suppl 2):1‐56. - PubMed
Bland 1997
Boissel 1999
-
- Boissel JP, Cucherat M, Li W, Chatellier G, Gueyffier F, Buyse M, et al. The problem of therapeutic efficacy indices, 3: comparison of the indices and their use [Apercu sur la problematique des indices d'efficacite therapeutique, 3: Comparaison des indices et utilisation. Groupe d'Etude des Indices D'efficacite]. Therapie 1999;54(4):405‐11. [PUBMED: 10667106] - PubMed
CEA
-
- Cost‐Effectiveness Analysis Registry (CEA). https://research.tufts‐nemc.org/cear4/ accessed 11/09/13.
Darling 1959
-
- Darling HF. Fluphenazine: a preliminary study. Disease of the Nervous System 1959;20(4):167‐70. - PubMed
Davies 2007
-
- Davies LM, Lewis S, Jones PB, Barnes TRE, Gaughran F, Hayhurst K, et al. Cost‐effectiveness of first‐ vs. second‐generation antipsychotic drugs: results from a randomised controlled trial in schizophrenia responding poorly to previous therapy. British Journal of Psychiatry 2007;191(JULY):14‐22. [EMBASE: 2007328135; MEDLINE: ] - PubMed
Deeks 2000
-
- Deeks J. Issues in the selection for meta‐analyses of binary data. Proceedings of the 8th International Cochrane Colloquium; 2000 Oct 25‐28; Cape Town. Cape Town: The Cochrane Collaboration, 2000.
Dencker 1988
-
- Dencker SJ, Johansson R, Malm U. Pharmacokinetic and pharmacodynamic studies on high doses of fluphenazine. Psychopharmacology (Berl) 1988;94(2):237‐41. - PubMed
Divine 1992
-
- Divine GW, Brown JT, Frazier LM. The unit of analysis error in studies about physicians' patient care behavior. Journal of General Internal Medicine 1992;7(6):623‐9. - PubMed
Donner 2002
-
- Donner A, Klar N. Issues in the meta‐analysis of cluster randomized trials. Statistics in Medicine 2002;21:2971‐80. - PubMed
Drummond 1996
Dysken 1981
-
- Dysken MW, Javaid JI, Chang SS, Schaffer C, Shahid A, Davis JM. Fluphenazine pharmacokinetics and therapeutic response. Psychopharmacology (Berl) 1981;73(3):205‐10. - PubMed
Egger 1997
Elbourne 2002
-
- Elbourne D, Altman DG, Higgins JPT, Curtina F, Worthingtond HV, Vaile A. Meta‐analyses involving cross‐over trials: methodological issues. International Journal of Epidemiology 2002;31(1):140‐9. - PubMed
Essali 2009
Evers 2005
-
- Evers S, Goossens M, Vet H, Tulder M, Ament A. Criteria list for assessment of methodological quality of economic evaluations: consensus on health economic criteria. International Journal of Technology Assessment in Health Care 2005;21(2 Spring):240‐5. - PubMed
Fleischhacker 1989
-
- Fleischhacker WW, Bergmann KJ, Perovich R, Pestreich LK, Borenstein M, Lieberman JA, et al. The Hillside Akathisia Scale: a new rating instrument for neuroleptic‐induced akathisia. Psychopharmacological Bulletin 1989;25(2):222‐6. - PubMed
Furukawa 2006
-
- Furukawa TA, Barbui C, Cipriani A, Brambilla P, Watanabe N. Imputing missing standard deviations in meta‐analyses can provide accurate results. Journal of Clinical Epidemiology 2006;59(7):7‐10. - PubMed
Gebhardt 1983
-
- Gebhardt R, Pietzcker A, Strauss A, Stoeckel M, Langer C, Freudenthal K. Scale formation in the AMDP‐system [Skalenbildung im AMDP‐System]. Archives of Psychiatry and Neurological Sciences 1983;233:223‐45. - PubMed
Grace 1991
-
- Grace AA. Phasic versus tonic dopamine release and the modulation of dopamine system responsivity: a hypothesis for the etiology of schizophrenia. Neuroscience 1991;41(1):1‐24. - PubMed
Gulliford 1999
-
- Gulliford MC. Components of variance and intraclass correlations for the design of community‐based surveys and intervention studies: data from the Health Survey for England 1994. American Journal of Epidemiology 1999;149:876‐83. - PubMed
Guy 1976
-
- Guy W. ECDEU Assessment Manual for Psychopharmacology. Rockville, MD: National Institute of Mental Health. DHEW Publication NO (ADM), 1976:124‐585.
Guy 1976a
-
- Guy E. Abnormal Involuntary Movement Scale. Rockville, MD, National Institute of Mental Health, U.S. Department of Health and Human Services: ECDEU Assessment Manual for Psychopharmacology 1976.
Healy 2012
HEED
-
- Health Economic Evaluation Database (HEED). Online ISBN: 9780470510933. [DOI: 10.1002/9780470510933] - DOI
HES 2012
-
- Hospital Episode Statistics, Admitted Patient Care ‐ England 2011‐12: Main Specialties (.xls). http://www.hscic.gov.uk/searchcatalogue?productid=9161&q=title%3a%22... (accessed May 2013) 2011‐12.
Higgins 2003
Higgins 2011
-
- Higgins JPT, Green S (editors). Cochrane Handbook for Systematic Reviews of Interventions Version 5.0.2 [updated September 2011]. The Cochrane Collaboration, 2011. Available from www.cochrane‐handbook.org.
Hogan 1983
-
- Hogan TP, Awad AG, Eastwood R. A self‐report scale predictive of drug compliance in schizophrenics: reliability and discriminative validity. Psychological Medicine 1983;13:177‐83. - PubMed
Hutton 2009
-
- Hutton JL. Number needed to treat and number needed to harm are not the best way to report and assess the results of randomised clinical trials. British Journal of Haematology 2009;146(1):27‐30. - PubMed
Jones 2006
-
- Jones PB, Barnes TRE, Davies L. Randomized controlled trial of the effect on quality of life of second‐ vs. first‐generation antipsychotic drugs in schizophrenia ‐ cost utility of the latest antipsychotic drugs in schizophrenia study (CUtLASS 1). Archives of General Psychiatry 2006;63:1079‐86. - PubMed
Kane 1986
-
- Kane JM, Woerner M, Sarantakos S. Depot neuroleptics: a comparison review of standard, intermediate and low‐dose regimens. Journal of Clinical Psychiatry 1986;47(Suppl 5):30‐3. - PubMed
Kay 1986
-
- Kay SR, Opler LA, Fiszbein A. Positive and Negative Syndrome Scale (PANSS) Manual. North Tonawanda, NY: Multi‐Health Systems, 1986.
Kay 1987
-
- Kay SR, Fiszbein A, Opler LA. The positive and negative syndrome scale (PANSS) for schizophrenia. Schizophrenia Bulletin 1987;13:261‐76. - PubMed
Kendall 2011
-
- Kendall T. The rise and fall of the atypical antipsychotics. British Journal of Psychiatry 2011;199:266‐8. - PubMed
Leucht 2005
-
- Leucht S, Kane JM, Kissling W, Hamann J, Etschel E, Engel RR. What does the PANSS mean?. Schizophrenia Research 2005;79(2‐3):231‐8. [PUBMED: 15982856] - PubMed
Leucht 2005a
-
- Leucht S, Kane JM, Kissling W, Hamann J, Etschel E, Engel R. Clinical implications of brief psychiatric rating scale scores. British Journal of Psychiatry 2005;187:366‐71. [PUBMED: 16199797] - PubMed
Leucht 2009
-
- Leucht S, Corves C, Arbter D, Engel RR, Li C, Davis JM. Second‐generation versus first‐generation antipsychotic drugs for schizophrenia: a meta‐analysis. Lancet 2009;373(9657):31‐41. - PubMed
Lieberman 2005
-
- Lieberman JA, Stroup TS, McEvoy JP. Effectiveness of antipsychotic drugs in patients with chronic schizophrenia. New England Journal of Medicine 2005;353:1209‐23. - PubMed
Maayan 2015
Maier 1988
Mangalore 2007
-
- Mangalore R, Knapp M. Cost of schizophrenia in England. Journal of Mental Health Policy and Economics 2007;10(1):23–41. - PubMed
Marshall 2000
-
- Marshall M, Lockwood A, Bradley C, Adams C, Joy C, Fenton M. Unpublished rating scales: a major source of bias in randomised controlled trials of treatments for schizophrenia. British Journal of Psychiatry 2000;176:249‐52. - PubMed
Marvaha 2004
-
- Marvaha S, Johnson S. Schizophrenia and employment ‐ a review. Social Psychiatry and Psychiatric Epidemiology 2004;39:337‐49. - PubMed
Matar 2013
McGrath 2008
-
- McGrath J, Saha S, Chant D, Welham J. Schizophrenia: a concise overview of incidence, prevalence, and mortality. Epidemiological Review 2008;30:67‐76. - PubMed
Millar 1963
-
- Millar J. A trial of fluphenazine in schizophrenia. British Journal of Psychiatry 1963;109:428‐32.
NICE 2012
-
- National Institute for Health and Clinical Excellence. The Epilepsies: the diagnosis and management of the epilepsies in adults and children in primary and secondary care (Pharmacological Update of Clinical Guideline 20). NCGC National Clinical Guideline Centre 2012:1‐636.
Overall 1962
-
- Overall JE, Gorham DR. The brief psychiatric rating scale. Psychological Reports 1962;10:799‐812.
Parrott 1980
-
- Parrott AC, Hindmarch I. The Leeds sleep evaluation questionnaire in psychopharmacological investigations ‐ a review. Psychopharmacology 1980;71:173‐9. - PubMed
PSSRU 2012
-
- Compiled by Lesley Curtis. Unit costs of health and social care 2012. http://www.pssru.ac.uk/project‐pages/unit‐costs/2012/ (accessed May 2013) 2012:47.
Saha 2005
Schünemann 2008
-
- Schünemann HJ, Oxman AD, Vist GE, Higgins JPT, Deeks JJ, Glasziou P, et al. Chapter 12: Interpreting results and drawing conclusions. In: Higgins JPT, Green S (editors) Cochrane Handbook for Systematic Reviews of Interventions. The Cochrane Collaboration, 2008. Available from www.cochrane‐handbook.org.
Seeman 2002
-
- Seeman P. Atypical antipsychotics: mechanism of action. Canadian Journal of Psychiatry 2002;47(1):27‐38. - PubMed
Simpson 1970
-
- Simpson GM, Angus JWS. A rating scale for extrapyramidal side effects. Acta Psychiatrica Scandanavica 1970;212:S11‐19. - PubMed
Tardy 2014
Ukoumunne 1999
-
- Ukoumunne OC, Gulliford MC, Chinn S, Sterne JAC, Burney PGJ. Methods for evaluating area‐wide and organisation‐based intervention in health and health care: a systematic review. Health Technology Assessment 1999;3(5):1‐75. - PubMed
van Os 2008
WHO 2005
-
- Essential Medicines: WHO model list. http://whqlibdoc.who.int/hq/2005/a87017_eng.pdf March 2005; Vol. 14th edition.
Williams 1987
-
- Williams, A (editor). Health economics: the cheerful face of dismal science?. Health and Economics. London (UK): Macmillan, 1987.
Xia 2009
-
- Xia J, Adams CE, Bhagat N, Bhagat V, Bhoopathi P, El‐Sayeh H, et al. Loss to outcomes stakeholder survey: the LOSS study. Psychiatric Bulletin 2009;33(7):254‐7.
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Research Materials
Miscellaneous

