Editor's Highlight: Subvisible Aggregates of Immunogenic Proteins Promote a Th1-Type Response
- PMID: 27370416
- PMCID: PMC5036615
- DOI: 10.1093/toxsci/kfw121
Editor's Highlight: Subvisible Aggregates of Immunogenic Proteins Promote a Th1-Type Response
Abstract
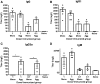
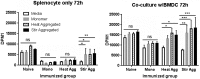
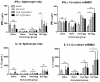
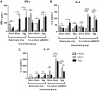
Protein aggregation is associated with enhanced immunogenicity of biotherapeutics. As a result, regulatory guidelines recommend screening for aggregation during bioprocessing. However, the mechanisms underlying the enhanced immunogenicity of aggregates are poorly understood. In the investigations described herein, the immunogenicity in mice of a humanized single chain variable antibody fragment (scFv) purified after expression in Escherichia coli has been examined. Reproducible scFv aggregates were obtained within the subvisible particle size range (mean diameter 2 µm) using thermal and mechanical stresses. Intraperitoneal immunization of BALB/c strain mice with 1 mg/ml of aggregated or monomeric scFv induced similar IgG and IgG1 antibody responses. In contrast, aggregate preparations stimulated significantly higher levels of anti-scFv IgG2a antibody than did the monomer. In comparative studies, aggregates of ovalbumin (OVA) within the subvisible particle size range were prepared by stir stress, and their immunogenicity compared with that of monomeric OVA in mice. Aggregated and monomeric OVA induced similar anti-OVA IgG and IgG1 antibody responses, whereas IgG2a antibody levels were significantly higher in aggregate-immunized mice. Furthermore, cytokine profiles in supernatants taken from splenocyte-dendritic cell co-cultures were consistent with aggregated preparations inducing a T helper (Th) 1-type response. Aggregated proteins within the subvisible range were therefore shown to induce a preferential Th1 type response, whereas monomeric proteins elicited a selective Th2 response. These data indicate that protein aggregation can impact on both the vigor and quality of immune responses.
Keywords: OVA.; biotherapeutic; immunogenicity; protein aggregation; scFv.
© The Author 2016. Published by Oxford University Press on behalf of the Society of Toxicology.
Figures




Similar articles
-
Immunological adjuvant effect of Boswellia serrata (BOS 2000) on specific antibody and cellular response to ovalbumin in mice.Int Immunopharmacol. 2011 Aug;11(8):968-75. doi: 10.1016/j.intimp.2011.02.011. Epub 2011 Mar 1. Int Immunopharmacol. 2011. PMID: 21371582
-
Influence of Escherichia coli chaperone DnaK on protein immunogenicity.Immunology. 2017 Mar;150(3):343-355. doi: 10.1111/imm.12689. Epub 2016 Dec 7. Immunology. 2017. PMID: 27859059 Free PMC article.
-
Dietary ribonucleotides modulate type 1 and type 2 T-helper cell responses against ovalbumin in young BALB/cJ mice.J Nutr. 2001 Apr;131(4):1165-70. doi: 10.1093/jn/131.4.1165. J Nutr. 2001. PMID: 11285320
-
Immunogenicity of therapeutic proteins: influence of aggregation.J Immunotoxicol. 2014 Apr-Jun;11(2):99-109. doi: 10.3109/1547691X.2013.821564. Epub 2013 Aug 6. J Immunotoxicol. 2014. PMID: 23919460 Free PMC article. Review.
-
Biophysical studies of amorphous protein aggregation and in vivo immunogenicity.Biophys Rev. 2022 Nov 23;14(6):1495-1501. doi: 10.1007/s12551-022-01011-y. eCollection 2022 Dec. Biophys Rev. 2022. PMID: 36465085 Free PMC article. Review.
Cited by
-
Optimizing a therapeutic humanized follicle-stimulating hormone-blocking antibody formulation by protein thermal shift assay.Ann N Y Acad Sci. 2023 Mar;1521(1):67-78. doi: 10.1111/nyas.14952. Epub 2023 Jan 11. Ann N Y Acad Sci. 2023. PMID: 36628526 Free PMC article.
-
Identification of B cell epitopes enhanced by protein unfolding and aggregation.Mol Immunol. 2019 Jan;105:181-189. doi: 10.1016/j.molimm.2018.11.020. Epub 2018 Dec 11. Mol Immunol. 2019. PMID: 30550980 Free PMC article.
-
Protein aggregation and immunogenicity of biotherapeutics.Int J Pharm. 2020 Jul 30;585:119523. doi: 10.1016/j.ijpharm.2020.119523. Epub 2020 Jun 9. Int J Pharm. 2020. PMID: 32531452 Free PMC article. Review.
-
PLM_Sol: predicting protein solubility by benchmarking multiple protein language models with the updated Escherichia coli protein solubility dataset.Brief Bioinform. 2024 Jul 25;25(5):bbae404. doi: 10.1093/bib/bbae404. Brief Bioinform. 2024. PMID: 39179250 Free PMC article.
-
Biodegradable PLGA-b-PEG Nanoparticles Induce T Helper 2 (Th2) Immune Responses and Sustained Antibody Titers via TLR9 Stimulation.Vaccines (Basel). 2020 May 29;8(2):261. doi: 10.3390/vaccines8020261. Vaccines (Basel). 2020. PMID: 32485944 Free PMC article.
References
-
- Abbas A. K., Murphy K. M., Sher A. (1996). Functional diversity of helper T lymphocytes. Nature 383, 787–793. - PubMed
-
- Abdolvahab M. H., Fazeli A., Halim A., Sediq A. S., Fazeli M. R., Schellekens H. (2016). Immunogenicity of recombinant human interferon beta-1b in immune-tolerant transgenic mice corresponds with the biophysical characteristics of aggregates. J. Interferon Cytokine Res. 36, doi:10.1089/jir.2015.0108. - PubMed
-
- Bartelds G. M., Krieckaert C. L., Nurmohamed M. T., van Schouwenburg P. A., Lems W. F., Twisk J. W., Dijkmans B. A., Aarden L., Wolbink G. L. (2011). Development of antidrug antibodies against adalimumab and association with disease activity and treatment failure during long-term follow-up. Jama 305, 1460–1468. - PubMed
-
- Bessa J., Boeckle S., Beck H., Buckel T., Schlicht S., Ebeling M., Kiialainen A., Koulov A., Boll B., Weiser T., et al. (2015). The immunogenicity of antibody aggregates in a novel transgenic mouse model. Pharmaceut. Res. 32, 2344–2359. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Research Materials

