The antioxidative defense system is involved in the premature senescence in transgenic tobacco (Nicotiana tabacum NC89)
- PMID: 27370650
- PMCID: PMC4930573
- DOI: 10.1186/s40659-016-0088-1
The antioxidative defense system is involved in the premature senescence in transgenic tobacco (Nicotiana tabacum NC89)
Abstract
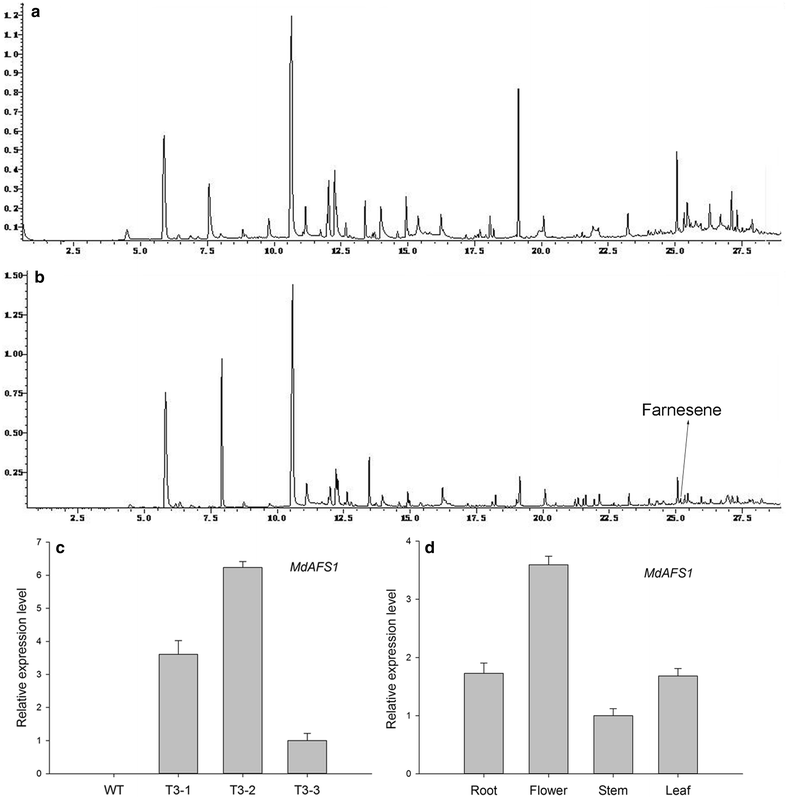
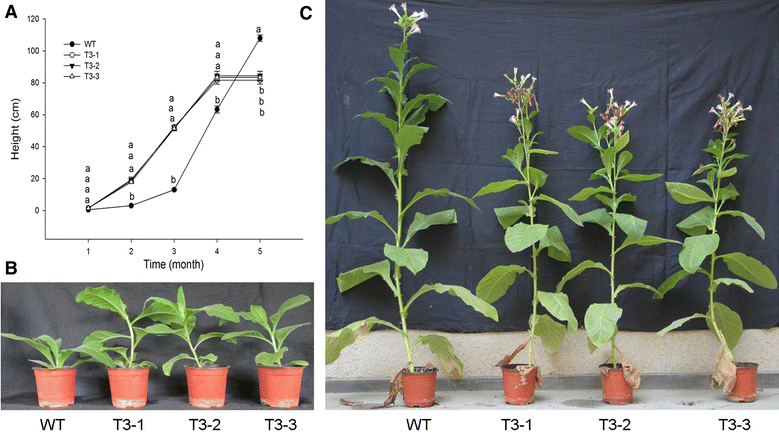
Background: α-Farnesene is a volatile sesquiterpene synthesized by the plant mevalonate (MVA) pathway through the action of α-farnesene synthase. The α-farnesene synthase 1 (MdAFS1) gene was isolated from apple peel (var. white winter pearmain), and transformed into tobacco (Nicotiana tabacum NC89). The transgenic plants had faster stem elongation during vegetative growth and earlier flowering than wild type (WT). Our studies focused on the transgenic tobacco phenotype.
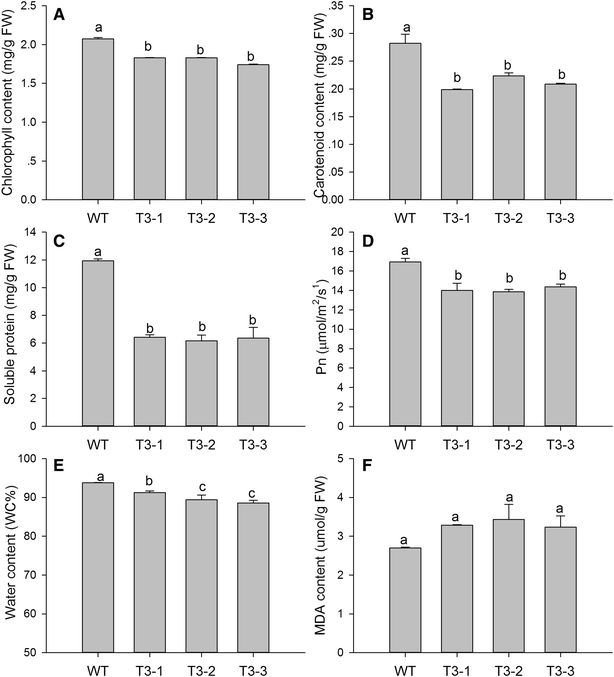
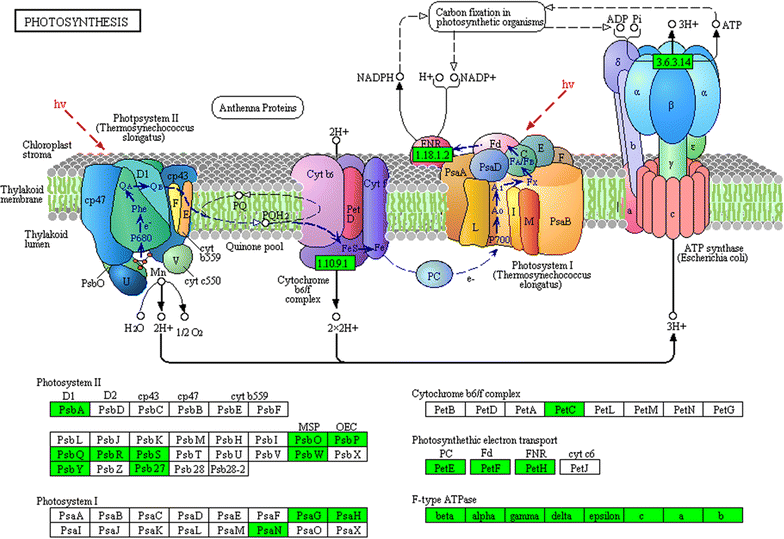
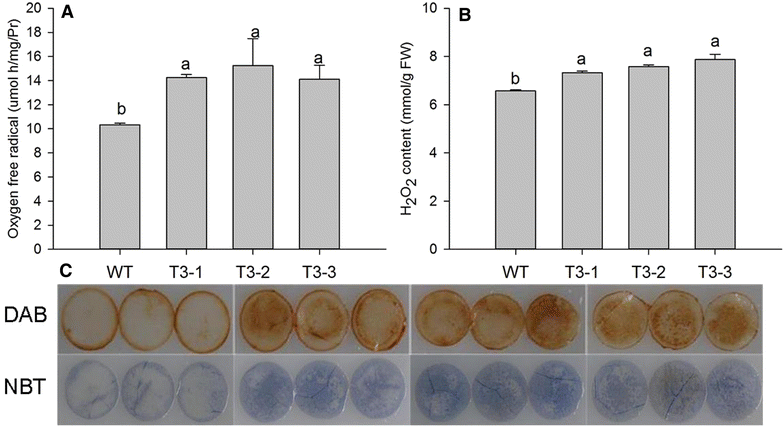
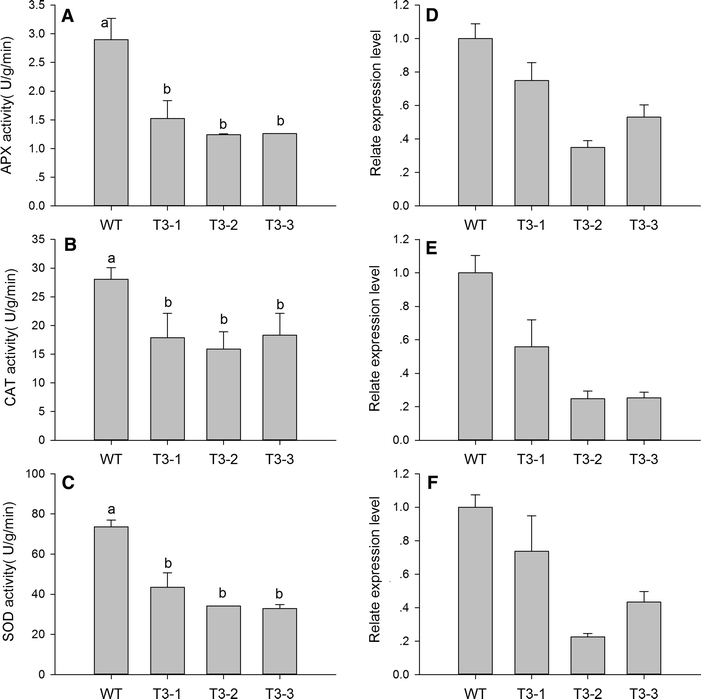
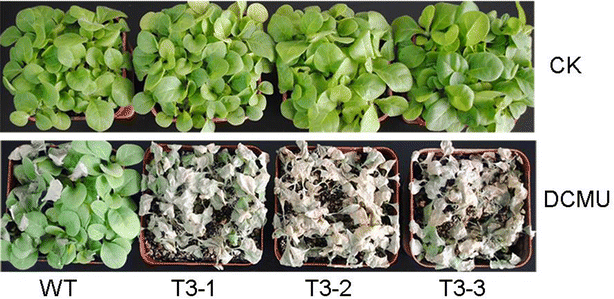
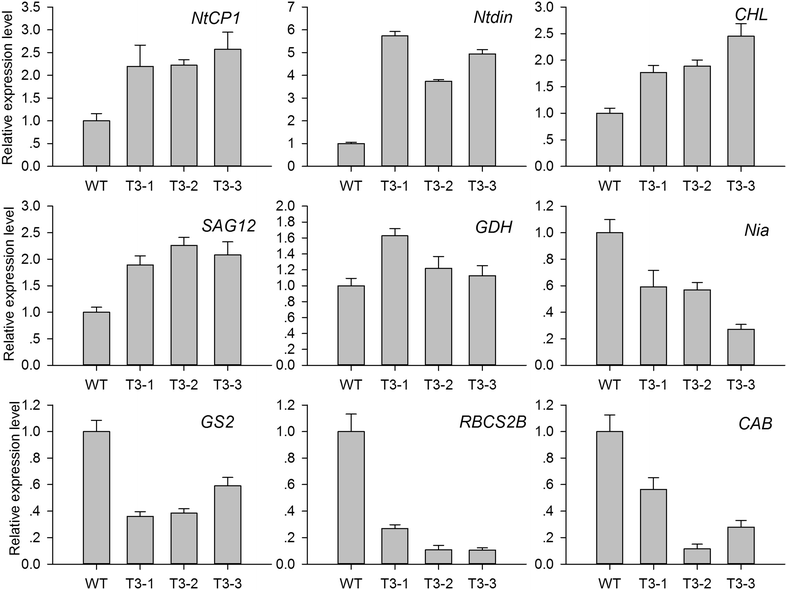
Results: The levels of chlorophyll and soluble protein decreased and a lower seed biomass and reduced net photosynthetic rate (Pn) in transgenic plants. Reactive oxygen species (ROS) such as hydrogen peroxide (H2O2) and superoxide radicals (O 2 (·-) ) had higher levels in transgenics compared to controls. Transgenic plants also had enhanced sensitivity to oxidative stress. The transcriptome of 8-week-old plants was studied to detect molecular changes. Differentially expressed unigene analysis showed that ubiquitin-mediated proteolysis, cell growth, and death unigenes were upregulated. Unigenes related to photosynthesis, antioxidant activity, and nitrogen metabolism were downregulated. Combined with the expression analysis of senescence marker genes, these results indicate that senescence started in the leaves of the transgenic plants at the vegetative growth stage.
Conclusions: The antioxidative defense system was compromised and the accumulation of reactive oxygen species (ROS) played an important role in the premature aging of transgenic plants.
Keywords: Reactive oxygen species (ROS); Senescence; Tobacco; Transcriptome.
Figures
Similar articles
-
Manipulation of monoubiquitin improves chilling tolerance in transgenic tobacco (Nicotiana tabacum).Plant Physiol Biochem. 2014 Feb;75:138-44. doi: 10.1016/j.plaphy.2013.11.003. Epub 2013 Nov 19. Plant Physiol Biochem. 2014. PMID: 24445300
-
De novo transcriptome assembly of transgenic tobacco (Nicotiana tabacum NC89) with early senescence characteristic.Physiol Mol Biol Plants. 2021 Feb;27(2):237-249. doi: 10.1007/s12298-021-00953-z. Epub 2021 Feb 19. Physiol Mol Biol Plants. 2021. PMID: 33707866 Free PMC article.
-
Tocopherol deficiency in transgenic tobacco (Nicotiana tabacum L.) plants leads to accelerated senescence.Plant Cell Environ. 2009 Feb;32(2):144-57. doi: 10.1111/j.1365-3040.2008.01907.x. Epub 2008 Nov 13. Plant Cell Environ. 2009. PMID: 19021891
-
Five ways to stay green.J Exp Bot. 2000 Feb;51 Spec No:329-37. doi: 10.1093/jexbot/51.suppl_1.329. J Exp Bot. 2000. PMID: 10938840 Review.
-
Recent advances in electrochemical detection of reactive oxygen species: a review.Analyst. 2025 Apr 8;150(8):1490-1517. doi: 10.1039/d4an01533a. Analyst. 2025. PMID: 40151998 Review.
Cited by
-
Expression of sucrose metabolizing enzymes in different sugarcane varieties under progressive heat stress.Front Plant Sci. 2023 Oct 16;14:1269521. doi: 10.3389/fpls.2023.1269521. eCollection 2023. Front Plant Sci. 2023. PMID: 37908828 Free PMC article.
-
The Roles of Environmental Factors in Regulation of Oxidative Stress in Plant.Biomed Res Int. 2019 May 8;2019:9732325. doi: 10.1155/2019/9732325. eCollection 2019. Biomed Res Int. 2019. PMID: 31205950 Free PMC article. Review.
-
Genome-wide identification and expression analysis of the R2R3-MYB gene family in tobacco (Nicotiana tabacum L.).BMC Genomics. 2022 Jun 9;23(1):432. doi: 10.1186/s12864-022-08658-7. BMC Genomics. 2022. PMID: 35681121 Free PMC article.
-
Antioxidant activation, cell wall reinforcement, and reactive oxygen species regulation promote resistance to waterlogging stress in hot pepper (Capsicum annuum L.).BMC Plant Biol. 2022 Sep 1;22(1):425. doi: 10.1186/s12870-022-03807-2. BMC Plant Biol. 2022. PMID: 36050651 Free PMC article.
-
Transcriptome Analysis of Gene Expression Patterns Potentially Associated with Premature Senescence in Nicotiana tabacum L.Molecules. 2018 Nov 2;23(11):2856. doi: 10.3390/molecules23112856. Molecules. 2018. PMID: 30400189 Free PMC article.
References
-
- Kännaste A, Vongvanich N, Borg-Karlson AK. Infestation by a Nalepella species induces emissions of α-and β-farnesenes,(−)-linalool and aromatic compounds in Norway spruce clones of different susceptibility to the large pine weevil. Arthropod-Plant Interact. 2008;2(1):31–41. doi: 10.1007/s11829-008-9029-4. - DOI
-
- Bain JM, Mercer FV. The Submicroscopio Cytology of Superficial Scald, a Physiological Disease of Apples. Aust J Biol Sci. 1963;16(2):442–449. doi: 10.1071/BI9630442. - DOI
-
- Meigh DF. Volatile compounds produced by apples. I.—Aldehydes and ketones. J Sci Food Agric. 1956;7(6):396–411. doi: 10.1002/jsfa.2740070604. - DOI
-
- Whitaker BD, Solomos T, Harrison DJ. Quantification of α-farnesene and its conjugated trienol oxidation products from apple peel by C18-HPLC with UV detection. J Agric Food Chem. 1997;45(3):760–765. doi: 10.1021/jf960780o. - DOI
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical

