Scan-o-matic: High-Resolution Microbial Phenomics at a Massive Scale
- PMID: 27371952
- PMCID: PMC5015956
- DOI: 10.1534/g3.116.032342
Scan-o-matic: High-Resolution Microbial Phenomics at a Massive Scale
Abstract
The capacity to map traits over large cohorts of individuals-phenomics-lags far behind the explosive development in genomics. For microbes, the estimation of growth is the key phenotype because of its link to fitness. We introduce an automated microbial phenomics framework that delivers accurate, precise, and highly resolved growth phenotypes at an unprecedented scale. Advancements were achieved through the introduction of transmissive scanning hardware and software technology, frequent acquisition of exact colony population size measurements, extraction of population growth rates from growth curves, and removal of spatial bias by reference-surface normalization. Our prototype arrangement automatically records and analyzes close to 100,000 growth curves in parallel. We demonstrate the power of the approach by extending and nuancing the known salt-defense biology in baker's yeast. The introduced framework represents a major advance in microbial phenomics by providing high-quality data for extensive cohorts of individuals and generating well-populated and standardized phenomics databases.
Keywords: genetics; high throughput; microbiology; phenomics; screening.
Copyright © 2016 Zackrisson et al.
Figures
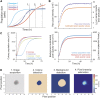

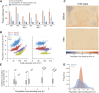

References
-
- Banks A. P., Lawless C., Lydall D. A., 2012. A quantitative fitness analysis workflow. J. Vis. Exp. 66 Available at: http://www.jove.com/video/4018/a-quantitative-fitness-analysis-workflow. - PMC - PubMed
-
- Blomberg A., 2011. Measuring growth rate in high-throughput growth phenotyping. Curr. Opin. Biotechnol. 22(1): 94–102. - PubMed
MeSH terms
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases
