Safety of Transcranial Direct Current Stimulation: Evidence Based Update 2016
- PMID: 27372845
- PMCID: PMC5007190
- DOI: 10.1016/j.brs.2016.06.004
Safety of Transcranial Direct Current Stimulation: Evidence Based Update 2016
Abstract
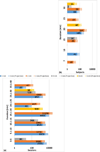
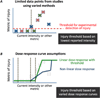
This review updates and consolidates evidence on the safety of transcranial Direct Current Stimulation (tDCS). Safety is here operationally defined by, and limited to, the absence of evidence for a Serious Adverse Effect, the criteria for which are rigorously defined. This review adopts an evidence-based approach, based on an aggregation of experience from human trials, taking care not to confuse speculation on potential hazards or lack of data to refute such speculation with evidence for risk. Safety data from animal tests for tissue damage are reviewed with systematic consideration of translation to humans. Arbitrary safety considerations are avoided. Computational models are used to relate dose to brain exposure in humans and animals. We review relevant dose-response curves and dose metrics (e.g. current, duration, current density, charge, charge density) for meaningful safety standards. Special consideration is given to theoretically vulnerable populations including children and the elderly, subjects with mood disorders, epilepsy, stroke, implants, and home users. Evidence from relevant animal models indicates that brain injury by Direct Current Stimulation (DCS) occurs at predicted brain current densities (6.3-13 A/m(2)) that are over an order of magnitude above those produced by conventional tDCS. To date, the use of conventional tDCS protocols in human trials (≤40 min, ≤4 milliamperes, ≤7.2 Coulombs) has not produced any reports of a Serious Adverse Effect or irreversible injury across over 33,200 sessions and 1000 subjects with repeated sessions. This includes a wide variety of subjects, including persons from potentially vulnerable populations.
Keywords: Electrical stimulation; Mood disorders; Safety; Transcranial Direct Current Stimulation; tDCS; tDCS safety.
Copyright © 2016 Elsevier Inc. All rights reserved.
Figures


Comment in
-
Response to letter to the editor: Safety of transcranial direct current stimulation: Evidence based update 2016.Brain Stimul. 2017 Sep-Oct;10(5):986-987. doi: 10.1016/j.brs.2017.06.007. Epub 2017 Jul 12. Brain Stimul. 2017. PMID: 28734680 Free PMC article. No abstract available.
-
Safety of transcranial direct current stimulation: Evidence based update 2016.Brain Stimul. 2017 Sep-Oct;10(5):983-985. doi: 10.1016/j.brs.2017.07.001. Epub 2017 Jul 8. Brain Stimul. 2017. PMID: 28751225 No abstract available.
References
-
- Sawyer DW, Donowitz GR, Mandell GL. Polymorphonuclear neutrophils: an effective antimicrobial force. Rev Infect Dis. 1989;11(Suppl 7):S1532–S1544. - PubMed
-
- Nitsche MA, Niehaus L, Hoffmann KT, Hengst S, Liebetanz D, Paulus W, et al. MRI study of human brain exposed to weak direct current stimulation of the frontal cortex. Clin Neurophysiol Off J Int Fed Clin Neurophysiol. 2004;115:2419–2423. - PubMed
Publication types
MeSH terms
Grants and funding
- P30 AG053760/AG/NIA NIH HHS/United States
- R25 GM056833/GM/NIGMS NIH HHS/United States
- I01 RX001534/RX/RRD VA/United States
- K01 HD078484/HD/NICHD NIH HHS/United States
- P30 AG028740/AG/NIA NIH HHS/United States
- UL1 TR001427/TR/NCATS NIH HHS/United States
- R03 EB017410/EB/NIBIB NIH HHS/United States
- R01 MH092926/MH/NIMH NIH HHS/United States
- R03 NS054783/NS/NINDS NIH HHS/United States
- KL2 TR000065/TR/NCATS NIH HHS/United States
- UL1 TR000064/TR/NCATS NIH HHS/United States
- R21 EB017510/EB/NIBIB NIH HHS/United States
- P20 GM109089/GM/NIGMS NIH HHS/United States
- UL1 TR000114/TR/NCATS NIH HHS/United States
- KL2 TR000113/TR/NCATS NIH HHS/United States
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical

