Benefits of commercial weight-loss programs on blood pressure and lipids: a systematic review
- PMID: 27373206
- PMCID: PMC5300307
- DOI: 10.1016/j.ypmed.2016.06.028
Benefits of commercial weight-loss programs on blood pressure and lipids: a systematic review
Abstract
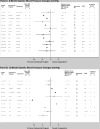
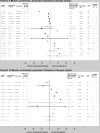
Our objective was to compare the effect of commercial weight-loss programs on blood pressure and lipids to control/education or counseling among individuals with overweight/obesity. We conducted a systematic review by searching MEDLINE and Cochrane Database of Systematic Reviews from inception to November 2014 and references identified by the programs. We included randomized, controlled trials ≥12weeks in duration. Two reviewers extracted information on study design, interventions, and mean change in systolic blood pressure (SBP), diastolic blood pressure (DBP), low-density lipoprotein cholesterol (LDL-c), high-density lipoprotein cholesterol (HDL-c), triglycerides, and total cholesterol and assessed risk of bias. We included 27 trials. Participants' blood pressure and lipids were normal at baseline in most trials. At 12months, Weight Watchers showed little change in blood pressure or lipid outcomes as compared to control/education (2 trials). At 12months, Atkins' participants had higher HDL-c and lower triglycerides than counseling (4 trials). Other programs had inconsistent effects or lacked long-term studies. Risk of bias was high for most trials of all programs. In conclusion, limited data exist regarding most commercial weight-loss programs' long-term effects on blood pressure and lipids. Clinicians should be aware that Weight Watchers has limited data that demonstrate CVD risk factor benefits relative to control/education. Atkins may be a reasonable option for patients with dyslipidemia. Additional well-designed, long-term trials are needed to confirm these conclusions and evaluate other commercial programs.
Keywords: Blood pressure; Cholesterol; Commercial weight-loss programs; Risk factors.
Copyright © 2016 Elsevier Inc. All rights reserved.
Conflict of interest statement
The authors have no personal conflicts of interest relevant to this article to disclose. Johns Hopkins University has an institutional consulting agreement with Healthways, Inc, which includes activities such as monitoring Innergy™, an employer-based commercial weight-loss intervention. Johns Hopkins receives fees for these services and royalty on the sales of this program.
Figures





References
-
- Flegal KM, Graubard BI, Williamson DF, Gail MH. Cause-specific excess deaths associated with underweight, overweight, and obesity. JAMA. 2007;298:2028–37. - PubMed
-
- Hubert HB, Feinleib M, McNamara PM, Castelli WP. Obesity as an independent risk factor for cardiovascular disease: a 26-year follow-up of participants in the Framingham Heart Study. Circulation. 1983;67:968–977. - PubMed
-
- Rexrode KM, Hennekens CH, Willett WC, Colditz GA, Stampfer MJ, Speizer FE, Manson JE. A prospective study of body mass index, weight change, and risk of stroke in women. JAMA. 1997;277:1539–1545. - PubMed
-
- Jensen MD, Ryan DH, Apovian CM, Ard JD, Comuzzie AG, Donato KA, Hu FB, Hubbard VS, Jakicic JM, Kushner RF, Loria CM, Millen BE, Nonas CA, Pi-Sunyer FX, Stevens J, Stevens VJ, Wadden TA, Wolfe BM, Yanovski SZ, Jordan HS, Kendall KA, Lux LJ, Mentor-Marcel R, Morgan LC, Trisolini MG, Wnek J, Anderson JL, Halperin JL, Albert NM, Bozkurt B, Brindis RG, Curtis LH, DeMets D, Hochman JS, Kovacs RJ, Ohman EM, Pressler SJ, Sellke FW, Shen WK, Smith SC, Jr, Tomaselli GF, American College of Cardiology/American Heart Association Task Force on Practice Guidelines 2013 AHA/ACC/TOS guideline for the management of overweight and obesity in adults: a report of the American College of Cardiology/American Heart Association Task Force on Practice Guidelines and The Obesity Society. Circulation. 2014;129:S102–38. - PMC - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical

