Chimeric antigen receptor-modified T cells for the treatment of solid tumors: Defining the challenges and next steps
- PMID: 27373504
- PMCID: PMC5035606
- DOI: 10.1016/j.pharmthera.2016.06.010
Chimeric antigen receptor-modified T cells for the treatment of solid tumors: Defining the challenges and next steps
Abstract
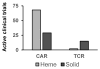
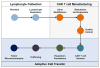
Chimeric antigen receptor (CAR) T cell therapy has shown promise in CD19 expressing hematologic malignancies, but how to translate this success to solid malignancies remains elusive. Effective translation of CAR T cells to solid tumors will require an understanding of potential therapeutic barriers, including factors that regulate CAR T cells expansion, persistence, trafficking, and fate within tumors. Herein, we describe the current state of CAR T cells in solid tumors; define key barriers to CAR T cell efficacy and mechanisms underlying these barriers, outline potential avenues for overcoming these therapeutic obstacles, and discuss the future of translating CAR T cells for the treatment of patients with solid malignancies.
Keywords: CAR T cells; Cellular immunity; Chimeric antigen receptor; Solid malignancies; Tumor microenvironment.
Copyright © 2016 Elsevier Inc. All rights reserved.
Conflict of interest statement
statement G.L.B. has received research funding from Novartis, Incyte, and Biothera. The authors declare no other conflicts of interest.
Figures
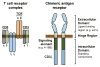
References
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources

