The role of microRNAs in respiratory viral infection: friend or foe?
- PMID: 27373545
- PMCID: PMC7169129
- DOI: 10.1002/rmv.1894
The role of microRNAs in respiratory viral infection: friend or foe?
Abstract
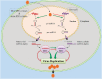
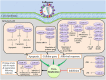
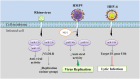
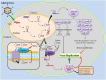
MicroRNAs (miRNAs) have emerged as a class of regulatory RNAs in host-pathogen interactions. Aberrant miRNA expression seems to play a central role in the pathology of several respiratory viruses, promoting development and progression of infection. miRNAs may thus serve as therapeutic and prognostic factors for respiratory viral infectious disease caused by a variety of agents. We present a comprehensive review of recent findings related to the role of miRNAs in different respiratory viral infections and discuss possible therapeutic opportunities aiming to attenuate the burden of viral infections. Our review supports the emerging concept that cellular and viral-encoded miRNAs might be broadly implicated in human respiratory viral infections, with either positive or negative effects on virus life cycle. Copyright © 2016 John Wiley & Sons, Ltd.
Copyright © 2016 John Wiley & Sons, Ltd.
Figures


References
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical

