Origin and Role of a Subset of Tumor-Associated Neutrophils with Antigen-Presenting Cell Features in Early-Stage Human Lung Cancer
- PMID: 27374224
- PMCID: PMC4945447
- DOI: 10.1016/j.ccell.2016.06.001
Origin and Role of a Subset of Tumor-Associated Neutrophils with Antigen-Presenting Cell Features in Early-Stage Human Lung Cancer
Abstract
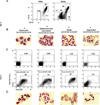
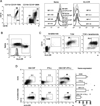
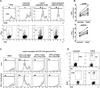
Based on studies in mouse tumor models, granulocytes appear to play a tumor-promoting role. However, there are limited data about the phenotype and function of tumor-associated neutrophils (TANs) in humans. Here, we identify a subset of TANs that exhibited characteristics of both neutrophils and antigen-presenting cells (APCs) in early-stage human lung cancer. These APC-like "hybrid neutrophils," which originate from CD11b(+)CD15(hi)CD10(-)CD16(low) immature progenitors, are able to cross-present antigens, as well as trigger and augment anti-tumor T cell responses. Interferon-γ and granulocyte-macrophage colony-stimulating factor are requisite factors in the tumor that, working through the Ikaros transcription factor, synergistically exert their APC-promoting effects on the progenitors. Overall, these data demonstrate the existence of a specialized TAN subset with anti-tumor capabilities in human cancer.
Copyright © 2016 Elsevier Inc. All rights reserved.
Conflict of interest statement
No conflict of interest exists.
Figures





Comment in
-
Tumor-Associated Neutrophils Show Phenotypic and Functional Divergence in Human Lung Cancer.Cancer Cell. 2016 Jul 11;30(1):11-13. doi: 10.1016/j.ccell.2016.06.016. Cancer Cell. 2016. PMID: 27411583
References
-
- Araki H, Katayama N, Yamashita Y, Mano H, Fujieda A, Usui E, Mitani H, Ohishi K, Nishii K, Masuya M, et al. Reprogramming of human postmitotic neutrophils into macrophages by growth factors. Blood. 2004;8:2973–2980. - PubMed
-
- Ashtekar AR, Saha B. Poly's plea: membership to the club of APCs. Trends Immunol. 2003;9:485–490. - PubMed
-
- Brandau S. The dichotomy of neutrophil granulocytes in cancer. Semin. Cancer Biol. 2013;3:139–140. - PubMed
-
- Carus A, Ladekarl M, Hager H, Pilegaard H, Nielsen PS, Donskov F. Tumor-associated neutrophils and macrophages in non-small cell lung cancer: no immediate impact on patient outcome. Lung Cancer. 2013;1:130–137. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Research Materials
Miscellaneous

