Activation of PPARγ by a Natural Flavonoid Modulator, Apigenin Ameliorates Obesity-Related Inflammation Via Regulation of Macrophage Polarization
- PMID: 27374313
- PMCID: PMC4972579
- DOI: 10.1016/j.ebiom.2016.06.017
Activation of PPARγ by a Natural Flavonoid Modulator, Apigenin Ameliorates Obesity-Related Inflammation Via Regulation of Macrophage Polarization
Abstract
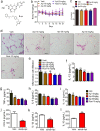
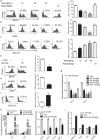
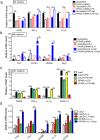
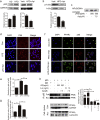
PPARγ has emerged as a master regulator of macrophage polarization and is the molecular target of the thiazolidinedione drugs. Here we show that apigenin binds and activates PPARγ by acting as a modulator. Activation of PPARγ by apigenin blocks p65 translocation into nuclei through inhibition of p65/PPARγ complex translocation into nuclei, thereby decreasing NF-κB activation and favoringM2 macrophage polarization. In HFD and ob/ob mice, apigenin significantly reverses M1 macrophage into M2 and reduces the infiltration of inflammatory cells in liver and adipose tissues, as well as decreases the levels of pro-inflammatory cytokines, thereby alleviating inflammation. Strikingly, apigenin reduces liver and muscular steatosis, decreases the levels of ALT, AST, TC and TG, improving glucose resistance obviously. Unlike rosiglitazone, apigenin does not cause significant weight gain, osteoporosis et al. Our findings identify apigenin as a modulator of PPARγ and a potential lead compound for treatment of metabolic disorders.
Keywords: Apigenin; Macrophage polarization; NF-κB; Obesity-related inflammation; PPARγ.
Copyright © 2016 The Authors. Published by Elsevier B.V. All rights reserved.
Figures




Similar articles
-
Adipogenic miR-27a in adipose tissue upregulates macrophage activation via inhibiting PPARγ of insulin resistance induced by high-fat diet-associated obesity.Exp Cell Res. 2017 Jun 15;355(2):105-112. doi: 10.1016/j.yexcr.2017.03.060. Epub 2017 Mar 30. Exp Cell Res. 2017. PMID: 28365247
-
Chrysin attenuates inflammation by regulating M1/M2 status via activating PPARγ.Biochem Pharmacol. 2014 Jun 15;89(4):503-14. doi: 10.1016/j.bcp.2014.03.016. Epub 2014 Apr 2. Biochem Pharmacol. 2014. PMID: 24704474
-
mTORC1 inhibition with rapamycin exacerbates adipose tissue inflammation in obese mice and dissociates macrophage phenotype from function.Immunobiology. 2017 Feb;222(2):261-271. doi: 10.1016/j.imbio.2016.09.014. Epub 2016 Sep 28. Immunobiology. 2017. PMID: 27692982
-
Adipose tissue macrophages.Immunol Lett. 2007 Oct 15;112(2):61-7. doi: 10.1016/j.imlet.2007.07.003. Epub 2007 Jul 31. Immunol Lett. 2007. PMID: 17719095 Review.
-
The "Big Bang" in obese fat: Events initiating obesity-induced adipose tissue inflammation.Eur J Immunol. 2015 Sep;45(9):2446-56. doi: 10.1002/eji.201545502. Epub 2015 Aug 19. Eur J Immunol. 2015. PMID: 26220361 Review.
Cited by
-
Functionalization with a Polyphenol-Rich Pomace Extract Empowers a Ceramic Bone Filler with In Vitro Antioxidant, Anti-Inflammatory, and Pro-Osteogenic Properties.J Funct Biomater. 2021 May 5;12(2):31. doi: 10.3390/jfb12020031. J Funct Biomater. 2021. PMID: 34063147 Free PMC article.
-
CD38 Is Robustly Induced in Human Macrophages and Monocytes in Inflammatory Conditions.Front Immunol. 2018 Jul 10;9:1593. doi: 10.3389/fimmu.2018.01593. eCollection 2018. Front Immunol. 2018. PMID: 30042766 Free PMC article.
-
Apigenin Prevents Acetaminophen-Induced Liver Injury by Activating the SIRT1 Pathway.Front Pharmacol. 2020 Apr 24;11:514. doi: 10.3389/fphar.2020.00514. eCollection 2020. Front Pharmacol. 2020. PMID: 32425778 Free PMC article.
-
Effect of modulation of PPAR-γ activity on Kupffer cells M1/M2 polarization in the development of non-alcoholic fatty liver disease.Sci Rep. 2017 Mar 16;7:44612. doi: 10.1038/srep44612. Sci Rep. 2017. PMID: 28300213 Free PMC article.
-
Desmodium styracifolium: Botanical and ethnopharmacological insights, phytochemical investigations, and prospects in pharmacology and pharmacotherapy.Heliyon. 2024 Jan 20;10(3):e25058. doi: 10.1016/j.heliyon.2024.e25058. eCollection 2024 Feb 15. Heliyon. 2024. PMID: 38317880 Free PMC article. Review.
References
-
- Audic S., Claverie J.M. The significance of digital gene expression profiles. Genome Res. 1997;7:986–995. - PubMed
-
- Bao X., Cui J., Wu Y., Han X., Gao C., Hua Z., Shen P. The roles of endogenous reactive oxygen species and nitric oxide in triptolide-induced apoptotic cell death in macrophages. J. Mol. Med. (Berl) 2007;85:85–98. - PubMed
-
- Bouhlel M.A., Derudas B., Rigamonti E., Dievart R., Brozek J., Haulon S., Zawadzki C., Jude B., Torpier G., Marx N., Staels B., Chinetti-Gbaguidi G. PPARgamma activation primes human monocytes into alternative M2 macrophages with anti-inflammatory properties. Cell Metab. 2007;6:137–143. - PubMed
-
- Buckingham R.E. Thiazolidinediones: pleiotropic drugs with potent anti-inflammatory properties for tissue protection. Hepatol. Res. 2005;33:167–170. - PubMed
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Miscellaneous

