Adjacent Codons Act in Concert to Modulate Translation Efficiency in Yeast
- PMID: 27374328
- PMCID: PMC4967012
- DOI: 10.1016/j.cell.2016.05.070
Adjacent Codons Act in Concert to Modulate Translation Efficiency in Yeast
Abstract
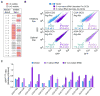
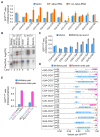
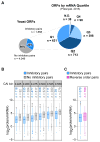
Translation elongation efficiency is largely thought of as the sum of decoding efficiencies for individual codons. Here, we find that adjacent codon pairs modulate translation efficiency. Deploying an approach in Saccharomyces cerevisiae that scored the expression of over 35,000 GFP variants in which three adjacent codons were randomized, we have identified 17 pairs of adjacent codons associated with reduced expression. For many pairs, codon order is obligatory for inhibition, implying a more complex interaction than a simple additive effect. Inhibition mediated by adjacent codons occurs during translation itself as GFP expression is restored by increased tRNA levels or by non-native tRNAs with exact-matching anticodons. Inhibition operates in endogenous genes, based on analysis of ribosome profiling data. Our findings suggest translation efficiency is modulated by an interplay between tRNAs at adjacent sites in the ribosome and that this concerted effect needs to be considered in predicting the functional consequences of codon choice.
Keywords: codon; ribosome; translation; yeast.
Copyright © 2016 Elsevier Inc. All rights reserved.
Conflict of interest statement
The authors declare no competing financial interests.
Figures



Similar articles
-
Translation of CGA codon repeats in yeast involves quality control components and ribosomal protein L1.RNA. 2013 Sep;19(9):1208-17. doi: 10.1261/rna.039446.113. Epub 2013 Jul 3. RNA. 2013. PMID: 23825054 Free PMC article.
-
Misdecoding of rare CGA codon by translation termination factors, eRF1/eRF3, suggests novel class of ribosome rescue pathway in S. cerevisiae.FEBS J. 2019 Feb;286(4):788-802. doi: 10.1111/febs.14709. Epub 2018 Dec 11. FEBS J. 2019. PMID: 30471181 Free PMC article.
-
Trade-offs between tRNA abundance and mRNA secondary structure support smoothing of translation elongation rate.Nucleic Acids Res. 2015 Mar 31;43(6):3022-32. doi: 10.1093/nar/gkv199. Epub 2015 Mar 12. Nucleic Acids Res. 2015. PMID: 25765653 Free PMC article.
-
Synonymous Codons: Choose Wisely for Expression.Trends Genet. 2017 Apr;33(4):283-297. doi: 10.1016/j.tig.2017.02.001. Epub 2017 Mar 12. Trends Genet. 2017. PMID: 28292534 Free PMC article. Review.
-
Regulated translational bypass of stop codons in yeast.Trends Microbiol. 2007 Feb;15(2):78-86. doi: 10.1016/j.tim.2006.12.002. Epub 2006 Dec 21. Trends Microbiol. 2007. PMID: 17187982 Review.
Cited by
-
Impaired 2',3'-cyclic phosphate tRNA repair causes thermo-sensitive genic male sterility in rice.Cell Res. 2024 Nov;34(11):763-775. doi: 10.1038/s41422-024-01012-4. Epub 2024 Sep 9. Cell Res. 2024. PMID: 39251844
-
Rqc1 and other yeast proteins containing highly positively charged sequences are not targets of the RQC complex.J Biol Chem. 2021 Jan-Jun;296:100586. doi: 10.1016/j.jbc.2021.100586. Epub 2021 Mar 24. J Biol Chem. 2021. PMID: 33774050 Free PMC article.
-
Ageing exacerbates ribosome pausing to disrupt cotranslational proteostasis.Nature. 2022 Jan;601(7894):637-642. doi: 10.1038/s41586-021-04295-4. Epub 2022 Jan 19. Nature. 2022. PMID: 35046576 Free PMC article.
-
The Dynamic Codon Biaser: calculating prokaryotic codon usage biases.Microb Genom. 2021 Oct;7(10):000663. doi: 10.1099/mgen.0.000663. Microb Genom. 2021. PMID: 34699346 Free PMC article.
-
How Messenger RNA and Nascent Chain Sequences Regulate Translation Elongation.Annu Rev Biochem. 2018 Jun 20;87:421-449. doi: 10.1146/annurev-biochem-060815-014818. Annu Rev Biochem. 2018. PMID: 29925264 Free PMC article. Review.
References
-
- Alexandrov A, Chernyakov I, Gu W, Hiley SL, Hughes TR, Grayhack EJ, Phizicky EM. Rapid tRNA decay can result from lack of nonessential modifications. Mol Cell. 2006;21:87–96. - PubMed
-
- Beggs JD. Transformation of yeast by a replicating hybrid plasmid. Nature. 1978;275:104–109. - PubMed
-
- Boycheva S, Chkodrov G, Ivanov I. Codon pairs in the genome of Escherichia coli. Bioinformatics. 2003;19:987–998. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases

