Compositional Control of Phase-Separated Cellular Bodies
- PMID: 27374333
- PMCID: PMC4967043
- DOI: 10.1016/j.cell.2016.06.010
Compositional Control of Phase-Separated Cellular Bodies
Abstract
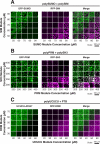
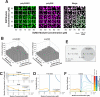
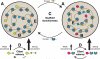
Cellular bodies such as P bodies and PML nuclear bodies (PML NBs) appear to be phase-separated liquids organized by multivalent interactions among proteins and RNA molecules. Although many components of various cellular bodies are known, general principles that define body composition are lacking. We modeled cellular bodies using several engineered multivalent proteins and RNA. In vitro and in cells, these scaffold molecules form phase-separated liquids that concentrate low valency client proteins. Clients partition differently depending on the ratio of scaffolds, with a sharp switch across the phase diagram diagonal. Composition can switch rapidly through changes in scaffold concentration or valency. Natural PML NBs and P bodies show analogous partitioning behavior, suggesting how their compositions could be controlled by levels of PML SUMOylation or cellular mRNA concentration, respectively. The data suggest a conceptual framework for considering the composition and control thereof of cellular bodies assembled through heterotypic multivalent interactions.
Copyright © 2016 Elsevier Inc. All rights reserved.
Figures




References
-
- Antolini F, Lo Bello M, Sette M. Purified promyelocytic leukemia coiled-coil aggregates as a tetramer displaying low α-helical content. Protein Expr. Purif. 2003;29:94–102. - PubMed
-
- Best JL, Ganiatsas S, Agarwal S, Changou A, Salomoni P, Shirihai O, Meluh PB, Pandolfi PP, Zon LI. SUMO-1 protease-1 regulates gene transcription through PML. Mol. Cell. 2002;10:843–855. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Research Materials
Miscellaneous

