Changing Balance of Spinal Cord Excitability and Nociceptive Brain Activity in Early Human Development
- PMID: 27374336
- PMCID: PMC4985558
- DOI: 10.1016/j.cub.2016.05.054
Changing Balance of Spinal Cord Excitability and Nociceptive Brain Activity in Early Human Development
Abstract
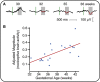
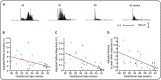
In adults, nociceptive reflexes and behavioral responses are modulated by a network of brain regions via descending projections to the spinal dorsal horn [1]. Coordinated responses to noxious inputs manifest from a balance of descending facilitation and inhibition. In contrast, young infants display exaggerated and uncoordinated limb reflexes [2]. Our understanding of nociceptive processing in the infant brain has been advanced by the use of electrophysiological and hemodynamic imaging [3-6]. From approximately 35 weeks' gestation, nociceptive-specific patterns of brain activity emerge [7], whereas prior to this, non-specific bursts of activity occur in response to noxious, tactile, visual, and auditory stimulation [7-10]. During the preterm period, refinement of spinal cord excitability is also observed: reflex duration shortens, response threshold increases, and improved discrimination between tactile and noxious events occurs [2, 11, 12]. However, the development of descending modulation in human infants remains relatively unexplored. In 40 infants aged 28-42 weeks' gestation, we examined the relationship between nociceptive brain activity and spinal reflex withdrawal activity in response to a clinically essential noxious procedure. Nociceptive-specific brain activity increases in magnitude with gestational age, whereas reflex withdrawal activity decreases in magnitude, duration, and latency across the same developmental period. By recording brain and spinal cord activity in the same infants, we demonstrate that the maturation of nociceptive brain activity is concomitant with the refinement of noxious-evoked limb reflexes. We postulate that, consistent with studies in animals, infant reflexes are influenced by the development of top-down inhibitory modulation from maturing subcortical and cortical brain networks.
Copyright © 2016 The Author(s). Published by Elsevier Ltd.. All rights reserved.
Figures


References
Publication types
MeSH terms
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources

