Itaconate Links Inhibition of Succinate Dehydrogenase with Macrophage Metabolic Remodeling and Regulation of Inflammation
- PMID: 27374498
- PMCID: PMC5108454
- DOI: 10.1016/j.cmet.2016.06.004
Itaconate Links Inhibition of Succinate Dehydrogenase with Macrophage Metabolic Remodeling and Regulation of Inflammation
Abstract
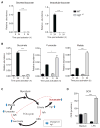
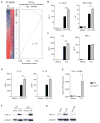
Remodeling of the tricarboxylic acid (TCA) cycle is a metabolic adaptation accompanying inflammatory macrophage activation. During this process, endogenous metabolites can adopt regulatory roles that govern specific aspects of inflammatory response, as recently shown for succinate, which regulates the pro-inflammatory IL-1β-HIF-1α axis. Itaconate is one of the most highly induced metabolites in activated macrophages, yet its functional significance remains unknown. Here, we show that itaconate modulates macrophage metabolism and effector functions by inhibiting succinate dehydrogenase-mediated oxidation of succinate. Through this action, itaconate exerts anti-inflammatory effects when administered in vitro and in vivo during macrophage activation and ischemia-reperfusion injury. Using newly generated Irg1(-/-) mice, which lack the ability to produce itaconate, we show that endogenous itaconate regulates succinate levels and function, mitochondrial respiration, and inflammatory cytokine production during macrophage activation. These studies highlight itaconate as a major physiological regulator of the global metabolic rewiring and effector functions of inflammatory macrophages.
Copyright © 2016 Elsevier Inc. All rights reserved.
Figures


References
-
- Ackermann WW, Potter VR. Enzyme inhibition in relation to chemotherapy. Proc Soc Exp Biol Med. 1949;72:1–9. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases

