Sam68 is a regulator of Toll-like receptor signaling
- PMID: 27374795
- PMCID: PMC5214940
- DOI: 10.1038/cmi.2016.32
Sam68 is a regulator of Toll-like receptor signaling
Abstract
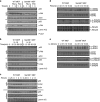
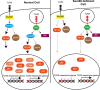
Recognition of pathogens by Toll-like receptors (TLR) activate multiple signaling cascades and expression of genes tailored to mount a primary immune response, inflammation, cell survival and apoptosis. Although TLR-induced activation of pathways, such as nuclear factor kappaB (NF-κB) and mitogen-activated protein kinases (MAPK), has been well studied, molecular entities controlling quantitative regulation of these pathways during an immune response remain poorly defined. We identified Sam68 as a novel regulator of TLR-induced NF-κB and MAPK activation. We found that TLR2 and TLR3 are totally dependent, whereas TLR4 is only partially dependent on Sam68 to induce the activation of NF-κB c-Rel. Absence of Sam68 greatly decreased TLR2- and TLR3-induced NF-κB p65 activation, whereas TLR4-induced p65 activation in a Sam68-independent manner. In contrast, Sam68 appeared to be a negative regulator of MAPK pathways because absence of Sam68 enhanced TLR2-induced activation of extracellular signal-regulated kinases (ERK) and c-Jun N-terminal kinases (JNK). Interestingly, TLR2- and TLR3-induced gene expression showed a differential requirement of Sam68. Absence of Sam68 impaired TLR2-induced gene expression, suggesting that Sam68 has a critical role in myeloid differentiation primary response gene 88-dependent TLR2 signaling. TLR3-induced gene expression that utilize Toll/Interleukin-1 receptor-domain-containing adapter-inducing beta interferon pathway, depend only partially on Sam68. Our findings suggest that Sam68 may function as an immune rheostat that balances the activation of NF-κB p65 and c-Rel, as well as MAPK, revealing a potential novel target to manipulate TLR signaling.
Figures



References
-
- Di Gioia M, Zanoni I. Toll-like receptor co-receptors as master regulators of the immune response. Mol Immunol 2015; 63: 143–152. - PubMed
-
- Takeda K, Akira S. Toll-like receptors in innate immunity. Int Immunol 2005; 17: 1–14. - PubMed
-
- Kanneganti TD, Lamkanfi M, Nunez G. Intracellular NOD-like receptors in host defense and disease. Immunity 2007; 27: 549–559. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Research Materials
Miscellaneous

