Oligo(lactic acid)n-Paclitaxel Prodrugs for Poly(ethylene glycol)-block-poly(lactic acid) Micelles: Loading, Release, and Backbiting Conversion for Anticancer Activity
- PMID: 27374999
- PMCID: PMC5576186
- DOI: 10.1021/jacs.6b03995
Oligo(lactic acid)n-Paclitaxel Prodrugs for Poly(ethylene glycol)-block-poly(lactic acid) Micelles: Loading, Release, and Backbiting Conversion for Anticancer Activity
Abstract
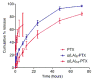
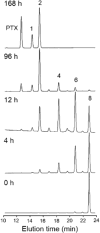
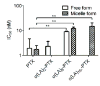
Poly(ethylene glycol)-block-poly(d,l-lactic acid) (PEG-b-PLA) micelles are nanocarriers for poorly water-soluble anticancer agents and have advanced paclitaxel (PTX) to humans due to drug solubilization, biocompatibility, and dose escalation. However, PEG-b-PLA micelles rapidly release PTX, resulting in widespread biodistribution and low tumor exposure. To improve delivery of PTX by PEG-b-PLA micelles, monodisperse oligo(l-lactic acid), o(LA)8 or o(LA)16, has been coupled onto PTX at the 7-OH position, forming ester prodrugs: o(LA)8-PTX and o(LA)16-PTX, respectively. As expected, o(LA)n-PTX was more compatible with PEG-b-PLA micelles than PTX, increasing drug loading from 11 to 54%. While in vitro release of PTX was rapid, resulting in precipitation, o(LA)n-PTX release was more gradual: t1/2 = 14 and 26 h for o(LA)8-PTX and o(LA)16-PTX, respectively. Notably, o(LA)8-PTX and o(LA)16-PTX in PEG-b-PLA micelles resisted backbiting chain end scission, based on reverse-phase HPLC analysis. By contrast, o(LA)8-PTX and o(LA)16-PTX degraded substantially in 1:1 acetonitrile:10 mM PBS, pH 7.4, at 37 °C, generating primarily o(LA)2-PTX. The IC50 value of o(LA)2-PTX was ∼2.3 nM for A549 human lung cancer cells, equipotent with PTX in vitro. After weekly IV injections at 20 mg/kg as PEG-b-PLA micelles, o(LA)8-PTX induced tumor regression in A549 tumor-bearing mice, whereas PTX delayed tumor growth. Surprisingly, o(LA)8-PTX caused less toxicity than PTX in terms of change in body weight. In conclusion, o(LA)n acts as a novel promoiety, undergoing backbiting conversion without a reliance on metabolizing enzymes, and o(LA)n-PTX improves PTX delivery by PEG-b-PLA micelles, providing a strong justification for clinical evaluation.
Conflict of interest statement
The authors declare no competing financial interests.
Figures


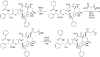
References
-
- Xiao RZ, Zeng ZW, Zhou GL, Wang JJ, Li FZ, Wang AM. Int J Nanomedicine. 2010;5:1057. - PMC - PubMed
- Kim SC, Kim DW, Shim YH, Bang JS, Oh HS, Kim SW, Seo MH. J Control Release. 2001;72:191–202. - PubMed
- Kim TY, Kim DW, Chung JY, Shin SG, Kim SC, Heo DS, Kim NK, Bang YJ. Clin Cancer Res. 2004;10:3708–3716. - PubMed
- Lee KS, Chung HC, Im SA, Park YH, Kim CS, Kim SB, Rha SY, Lee MY, Ro J. Breast Cancer Res Treat. 2008;108:241–250. - PubMed
-
- Cho H, Gao J, Kwon GS. J Control Release. 2015 in press. http://dx.doi.org/10.1016/j.jconrel.2015.12.015. - DOI - PubMed
-
- Ma X, Huang X, Huang G, Li L, Wang Y, Luo X, Boothman DA, Gao J. Adv Healthc Mater. 2014;3:1210–1216. - PMC - PubMed
- Miller T, Breyer S, van Colen G, Mier W, Haberkorn U, Geissler S, Voss S, Weigandt M, Goepferich A. Int J Pharm. 2013;445:117–124. - PubMed
- Owen SC, Chan DP, Shoichet MS. Nano Today. 2012;7:53–65.
-
- Ansell SM, Johnstone SA, Tardi PG, Lo L, Xie S, Shu Y, Harasym TO, Harasym NL, Williams L, Bermudes D, Liboiron BD, Saad W, Prud’homme RK, Mayer LD. J Med Chem. 2008;51:3288–3296. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Research Materials

