Safety and durability of effect of contralateral-eye administration of AAV2 gene therapy in patients with childhood-onset blindness caused by RPE65 mutations: a follow-on phase 1 trial
- PMID: 27375040
- PMCID: PMC5351775
- DOI: 10.1016/S0140-6736(16)30371-3
Safety and durability of effect of contralateral-eye administration of AAV2 gene therapy in patients with childhood-onset blindness caused by RPE65 mutations: a follow-on phase 1 trial
Abstract
Background: Safety and efficacy have been shown in a phase 1 dose-escalation study involving a unilateral subretinal injection of a recombinant adeno-associated virus (AAV) vector containing the RPE65 gene (AAV2-hRPE65v2) in individuals with inherited retinal dystrophy caused by RPE65 mutations. This finding, along with the bilateral nature of the disease and intended use in treatment, prompted us to determine the safety of administration of AAV2-hRPE65v2 to the contralateral eye in patients enrolled in the phase 1 study.
Methods: In this follow-on phase 1 trial, one dose of AAV2-hRPE65v2 (1.5 × 10(11) vector genomes) in a total volume of 300 μL was subretinally injected into the contralateral, previously uninjected, eyes of 11 children and adults (aged 11-46 years at second administration) with inherited retinal dystrophy caused by RPE65 mutations, 1.71-4.58 years after the initial subretinal injection. We assessed safety, immune response, retinal and visual function, functional vision, and activation of the visual cortex from baseline until 3 year follow-up, with observations ongoing. This study is registered with ClinicalTrials.gov, number NCT01208389.
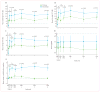
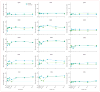
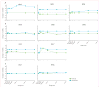
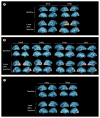
Findings: No adverse events related to the AAV were reported, and those related to the procedure were mostly mild (dellen formation in three patients and cataracts in two). One patient developed bacterial endophthalmitis and was excluded from analyses. We noted improvements in efficacy outcomes in most patients without significant immunogenicity. Compared with baseline, pooled analysis of ten participants showed improvements in mean mobility and full-field light sensitivity in the injected eye by day 30 that persisted to year 3 (mobility p=0.0003, white light full-field sensitivity p<0.0001), but no significant change was seen in the previously injected eyes over the same time period (mobility p=0.7398, white light full-field sensitivity p=0.6709). Changes in visual acuity from baseline to year 3 were not significant in pooled analysis in the second eyes or the previously injected eyes (p>0.49 for all time-points compared with baseline).
Interpretation: To our knowledge, AAV2-hRPE65v2 is the first successful gene therapy administered to the contralateral eye. The results highlight the use of several outcome measures and help to delineate the variables that contribute to maximal benefit from gene augmentation therapy in this disease.
Funding: Center for Cellular and Molecular Therapeutics at The Children's Hospital of Philadelphia, Spark Therapeutics, US National Institutes of Health, Foundation Fighting Blindness, Institute for Translational Medicine and Therapeutics, Research to Prevent Blindness, Center for Advanced Retinal and Ocular Therapeutics, Mackall Foundation Trust, F M Kirby Foundation, and The Research Foundation-Flanders.
Copyright © 2016 Elsevier Ltd. All rights reserved.
Conflict of interest statement
AMM and JB are co-inventors on a patent for “a method of treating or retarding the development of blindness” (US Patent number 8147823) but waived any potential financial interest in this technology in 2002. KAH, JFW, DCC, and JW are now employed by and have equity in Spark Therapeutics, a company that was formed after the participants had received intervention to the second eye and that is developing this technology. JB also reports having served on a scientific advisory board for Avalanche Technologies and is a founder of Gensight Biologics. JB, DCC, AMM, KAH, JW, KAM, SM, and JS are coauthors of a provisional patent describing the mobility test used in this study. FM and KAH hold a patent on methods for detection and modulation of T-cell responses to gene therapy vectors. JFW is an inventor on patents relative to adeno-associated vector development. All other authors declare no competing interests.
Figures



Comment in
-
Benefits of gene therapy for both eyes.Lancet. 2016 Aug 13;388(10045):635-6. doi: 10.1016/S0140-6736(16)30783-8. Epub 2016 Jun 30. Lancet. 2016. PMID: 27375039 No abstract available.
-
Vision quest: gene therapy for inherited vision loss.Lancet. 2018 Jan 6;391(10115):2. doi: 10.1016/S0140-6736(18)30004-7. Lancet. 2018. PMID: 29323644 No abstract available.
References
-
- Thompson DA, Gyurus P, Fleischer LL, et al. Genetics and phenotypes of RPE65 mutations in inherited retinal degeneration. Invest Ophthalmol Vis Sci. 2000;41:4293–99. - PubMed
-
- Redmond TM, Yu S, Lee E, et al. Rpe65 is necessary for production of 11-cis-vitamin A in the retinal visual cycle. Nat Genet. 1998;20:344–51. - PubMed
-
- Acland GM, Aguirre GD, Ray J, et al. Gene therapy restores vision in a canine model of childhood blindness. Nat Genet. 2001;28:92–95. - PubMed
Publication types
MeSH terms
Substances
Associated data
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical

